Welcome To Roj Bash Kurdistan


Coronavirus: we separate myths from facts and give advice
Re: Coronavirus: we separate myths from facts and give advic
UK post lock-down plans
Gyms and non-essential shops in all areas are expected to be allowed to reopen when England's lockdown ends
On Monday afternoon, Boris Johnson will explain the detail of England's return to the "three-tier system" when lockdown ends on 2 December.
It is reported pubs in tier three will stay shut except for takeaway. In tier two, only those serving meals can open.
Last orders in all pubs will remain at 22:00 GMT, but customers will have an extra hour to drink up.
The ban on outdoor grassroots sport is also set to be lifted in all tiers, following calls for this restriction to be eased.
And mass testing will be introduced in all tier three areas.
Details of which tier every region of England will be put into are expected on Thursday.
More areas are set to be placed in the higher tiers - high risk or very high risk - after lock-down.
Gyms and non-essential shops have been closed in England since 5 November, but are expected to reopen in all areas. Gyms were previously allowed to open in tier three, despite initially being told to shut in some places.
What will we be allowed to do at Christmas?
The prime minister had hoped to announce arrangements for the Christmas period on Monday, but this has been delayed until at least Tuesday to allow the Scottish and Welsh governments to agree the plans.
It comes after the Westminster government said the UK's four nations had backed plans to allow some household mixing "for a small number of days" over Christmas.
One option that was discussed in meetings this weekend was that three households could be allowed to meet up for up to five days, according to the BBC's deputy political editor Vicki Young.
Health Secretary Matt Hancock told BBC Radio 4's Today programme the government hoped to agree a "cautious, balanced approach" for Christmas "that can allow people to see their families, but also makes sure that we can keep the virus under control".
Coronavirus in the UK
Mr Johnson is expected to tell the House of Commons later: "The selflessness of people in following the rules is making a difference."
The increase in new cases is "flattening off" in England following the introduction of the nationwide lock-down measures, he will say.
The prime minister will say "we are not out of the woods yet", with the virus "both far more infectious and far more deadly than seasonal flu".
"But with expansion in testing and vaccines edging closer to deployment, the regional tiered system will help get the virus back under control and keep it there," he will say.
Cases chart
The plan for extensive community testing in tier three areas follows a pilot programme in Liverpool, where more than 200,000 people were tested and which the government said contributed to the fall in cases there.
Mr Johnson is expected to tell MPs that rapid testing will "help get the virus back under control and keep it there".
Daily coronavirus tests will be offered to close contacts of people who have tested positive in England, as a way to reduce the current 14-day quarantine.
Downing Street also said weekly testing would be expanded to all staff working in food manufacturing, prisons and the vaccine programme from next month.
On Sunday, the UK recorded another 18,662 confirmed coronavirus cases and 398 deaths within 28 days of a positive test. The total includes 141 deaths which were omitted from the 21 November figures in error.
https://www.bbc.co.uk/news/uk-55038155
Gyms and non-essential shops in all areas are expected to be allowed to reopen when England's lockdown ends
On Monday afternoon, Boris Johnson will explain the detail of England's return to the "three-tier system" when lockdown ends on 2 December.
It is reported pubs in tier three will stay shut except for takeaway. In tier two, only those serving meals can open.
Last orders in all pubs will remain at 22:00 GMT, but customers will have an extra hour to drink up.
The ban on outdoor grassroots sport is also set to be lifted in all tiers, following calls for this restriction to be eased.
And mass testing will be introduced in all tier three areas.
Details of which tier every region of England will be put into are expected on Thursday.
More areas are set to be placed in the higher tiers - high risk or very high risk - after lock-down.
Gyms and non-essential shops have been closed in England since 5 November, but are expected to reopen in all areas. Gyms were previously allowed to open in tier three, despite initially being told to shut in some places.
What will we be allowed to do at Christmas?
The prime minister had hoped to announce arrangements for the Christmas period on Monday, but this has been delayed until at least Tuesday to allow the Scottish and Welsh governments to agree the plans.
It comes after the Westminster government said the UK's four nations had backed plans to allow some household mixing "for a small number of days" over Christmas.
One option that was discussed in meetings this weekend was that three households could be allowed to meet up for up to five days, according to the BBC's deputy political editor Vicki Young.
Health Secretary Matt Hancock told BBC Radio 4's Today programme the government hoped to agree a "cautious, balanced approach" for Christmas "that can allow people to see their families, but also makes sure that we can keep the virus under control".
Coronavirus in the UK
Mr Johnson is expected to tell the House of Commons later: "The selflessness of people in following the rules is making a difference."
The increase in new cases is "flattening off" in England following the introduction of the nationwide lock-down measures, he will say.
The prime minister will say "we are not out of the woods yet", with the virus "both far more infectious and far more deadly than seasonal flu".
"But with expansion in testing and vaccines edging closer to deployment, the regional tiered system will help get the virus back under control and keep it there," he will say.
Cases chart
The plan for extensive community testing in tier three areas follows a pilot programme in Liverpool, where more than 200,000 people were tested and which the government said contributed to the fall in cases there.
Mr Johnson is expected to tell MPs that rapid testing will "help get the virus back under control and keep it there".
Daily coronavirus tests will be offered to close contacts of people who have tested positive in England, as a way to reduce the current 14-day quarantine.
Downing Street also said weekly testing would be expanded to all staff working in food manufacturing, prisons and the vaccine programme from next month.
On Sunday, the UK recorded another 18,662 confirmed coronavirus cases and 398 deaths within 28 days of a positive test. The total includes 141 deaths which were omitted from the 21 November figures in error.
https://www.bbc.co.uk/news/uk-55038155
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Oxford University vaccine
is highly effective
The Oxford/AstraZeneca vaccine is currently in the final stages of testing
The coronavirus vaccine developed by the University of Oxford is highly effective at stopping people developing Covid-19 symptoms, a large trial shows.
Overall results showed 70% protection, but the researchers say the figure may be as high as 90% by tweaking the dose.
The results will be seen as a triumph, but also come off the back of Pfizer and Moderna showing 95% protection.
However, the Oxford jab is far cheaper, and is easier to store and get to every corner of the world than the other two.
So the vaccine will play a significant role in tackling the pandemic, if it is approved for use by regulators.
"The announcement today takes us another step closer to the time when we can use vaccines to bring an end to the devastation caused by [the virus]," said the vaccine's architect Prof Sarah Gilbert.
The UK government has pre-ordered 100 million doses of the Oxford vaccine and AstraZeneca says it will make three billion doses for the world next year.
Prime Minister Boris Johnson said it was "incredibly exciting news" and that while there were still safety checks to come, "these are fantastic results".
The vaccine has been developed in around 10 months, a process that normally takes a decade.
What did the trial show?
More than 20,000 volunteers were involved, half in the UK, the rest in Brazil.
There were 30 cases of Covid in people who had two doses of the vaccine and 101 cases in people who received a dummy injection.
When volunteers were given two "high" doses the protection was 62%, but this rose to 90% when people were given a "low" dose followed by a high one. It's not clear why there is a difference.
"We're really pleased with these results," Prof Andrew Pollard, the trial's lead investigator, told the BBC.
He said the 90% effectiveness data was "intriguing" and would mean "we would have a lot more doses to distribute."
There were also lower levels of asymptomatic infection in the low-followed-by-high-dose group which "means we might be able to halt the virus in its tracks," Prof Pollard said.
When will I get it?
In the UK there are four million doses ready to go, with another 96 million to be delivered.
But nothing can happen until the vaccine has been approved by regulators who will assess the vaccine's safety, effectiveness, and that it is manufactured to high standard. This process will happen in the coming weeks.
However, the UK is ready to press the go button on an unprecedented mass immunisation campaign that dwarfs either the annual flu or childhood vaccination programmes.
Care home residents and staff will be first in the queue, followed by healthcare workers and the over-80s. The plan is to then to work down through the age groups.
How does it work?
The vaccine is a genetically modified common cold virus that used to infect chimpanzees.
It has been altered to stop it causing an infection in people and to carry the blueprints for part of the coronavirus, known as the spike protein.
Once these blueprints are inside the body they start the producing the coronavirus' spike protein, which the immune system recognizes as a threat and tries to squash it.
How the coronavirus vaccine works:
After Pfizer and Moderna both produced vaccines delivering 95% protection from Covid-19, a figure of 70% will be seen by some as relatively disappointing.
However, anything above 50% would have been considered a triumph just a month ago and 70% is comfortably better than the seasonal flu jab.
This is still a vaccine that can save lives from Covid-19.
It also has crucial advantages that make it easier to use. It can be stored at fridge temperature, which means it can be distributed to every corner of the world, unlike the Pfizer/BioNTech and Moderna vaccines, which need to be stored at much colder temperatures.
Oxford's manufacturing partner, AstraZeneca, is preparing to make three billion doses worldwide.
The Oxford vaccine, at a price of around £3, also costs far less than Pfizer's (around £15) or Moderna's (£25) vaccines.
What difference will this make to my life?
A vaccine is what we've spent the year waiting for and what lockdowns have bought time for.
However, producing enough vaccine and then immunising tens of millions of people in the UK, and billions around the world, is still a gargantuan challenge.
Life will not return to normal tomorrow, but the situation could improve dramatically as those most at risk are protected.
Health Secretary Matt Hancock told BBC Breakfast we would be "something closer to normal" by the summer but "until we can get that vaccine rolled out, we all need to look after each other".
What's the reaction been?
Prof Peter Horby, from the University of Oxford by not involved in the trial, said: "This is very welcome news, we can clearly see the end of tunnel now. There were no Covid hospitalisations or deaths in people who got the Oxford vaccine."
Dr Stephen Griffin, from the University of Leeds, said: "This is yet more excellent news and should be considered tremendously exciting. It has great potential to be delivered across the globe, achieving huge public health benefits.
https://www.bbc.co.uk/news/health-55040635
is highly effective
The Oxford/AstraZeneca vaccine is currently in the final stages of testing
The coronavirus vaccine developed by the University of Oxford is highly effective at stopping people developing Covid-19 symptoms, a large trial shows.
Overall results showed 70% protection, but the researchers say the figure may be as high as 90% by tweaking the dose.
The results will be seen as a triumph, but also come off the back of Pfizer and Moderna showing 95% protection.
However, the Oxford jab is far cheaper, and is easier to store and get to every corner of the world than the other two.
So the vaccine will play a significant role in tackling the pandemic, if it is approved for use by regulators.
"The announcement today takes us another step closer to the time when we can use vaccines to bring an end to the devastation caused by [the virus]," said the vaccine's architect Prof Sarah Gilbert.
The UK government has pre-ordered 100 million doses of the Oxford vaccine and AstraZeneca says it will make three billion doses for the world next year.
Prime Minister Boris Johnson said it was "incredibly exciting news" and that while there were still safety checks to come, "these are fantastic results".
The vaccine has been developed in around 10 months, a process that normally takes a decade.
What did the trial show?
More than 20,000 volunteers were involved, half in the UK, the rest in Brazil.
There were 30 cases of Covid in people who had two doses of the vaccine and 101 cases in people who received a dummy injection.
- WHOOP-DE-DO
An entire 20,000 people

Think I will wait for a lot more testing

Suggest vaccine is tested on murderers, rapists, pedophiles, pimps and drug dealers
All of the above are expendable

Much rather drugs tested on them and NOT innocent animals

When volunteers were given two "high" doses the protection was 62%, but this rose to 90% when people were given a "low" dose followed by a high one. It's not clear why there is a difference.
"We're really pleased with these results," Prof Andrew Pollard, the trial's lead investigator, told the BBC.
He said the 90% effectiveness data was "intriguing" and would mean "we would have a lot more doses to distribute."
There were also lower levels of asymptomatic infection in the low-followed-by-high-dose group which "means we might be able to halt the virus in its tracks," Prof Pollard said.
When will I get it?
In the UK there are four million doses ready to go, with another 96 million to be delivered.
But nothing can happen until the vaccine has been approved by regulators who will assess the vaccine's safety, effectiveness, and that it is manufactured to high standard. This process will happen in the coming weeks.
However, the UK is ready to press the go button on an unprecedented mass immunisation campaign that dwarfs either the annual flu or childhood vaccination programmes.
Care home residents and staff will be first in the queue, followed by healthcare workers and the over-80s. The plan is to then to work down through the age groups.
How does it work?
The vaccine is a genetically modified common cold virus that used to infect chimpanzees.
It has been altered to stop it causing an infection in people and to carry the blueprints for part of the coronavirus, known as the spike protein.
Once these blueprints are inside the body they start the producing the coronavirus' spike protein, which the immune system recognizes as a threat and tries to squash it.
How the coronavirus vaccine works:
- The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans.
Scientists then added genes for the spike surface protein of the coronavirus. This should prompt the immune system to produce neutralising antibodies, which would recognise and prevent any future coronavirus infection.
When the immune system comes into contact with the virus for real, it now knows what to do.
After Pfizer and Moderna both produced vaccines delivering 95% protection from Covid-19, a figure of 70% will be seen by some as relatively disappointing.
However, anything above 50% would have been considered a triumph just a month ago and 70% is comfortably better than the seasonal flu jab.
This is still a vaccine that can save lives from Covid-19.
It also has crucial advantages that make it easier to use. It can be stored at fridge temperature, which means it can be distributed to every corner of the world, unlike the Pfizer/BioNTech and Moderna vaccines, which need to be stored at much colder temperatures.
Oxford's manufacturing partner, AstraZeneca, is preparing to make three billion doses worldwide.
The Oxford vaccine, at a price of around £3, also costs far less than Pfizer's (around £15) or Moderna's (£25) vaccines.
What difference will this make to my life?
A vaccine is what we've spent the year waiting for and what lockdowns have bought time for.
However, producing enough vaccine and then immunising tens of millions of people in the UK, and billions around the world, is still a gargantuan challenge.
Life will not return to normal tomorrow, but the situation could improve dramatically as those most at risk are protected.
Health Secretary Matt Hancock told BBC Breakfast we would be "something closer to normal" by the summer but "until we can get that vaccine rolled out, we all need to look after each other".
What's the reaction been?
Prof Peter Horby, from the University of Oxford by not involved in the trial, said: "This is very welcome news, we can clearly see the end of tunnel now. There were no Covid hospitalisations or deaths in people who got the Oxford vaccine."
Dr Stephen Griffin, from the University of Leeds, said: "This is yet more excellent news and should be considered tremendously exciting. It has great potential to be delivered across the globe, achieving huge public health benefits.
https://www.bbc.co.uk/news/health-55040635
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Oxford/AstraZeneca
vaccine dose error
On Monday, the world heard how the UK's Covid vaccine - from AstraZeneca and Oxford University - was highly effective in advanced trials
It gave hope of another new jab to fight the pandemic that should be cheaper and easier to distribute than the Pfizer/BioNTech and Moderna mRNA vaccines that announced similarly impressive results just days before.
But after the jubilation, some negative press has followed.
On Thursday, multiple news outlets in the UK and US reported that there were questions over the data. They weren't about safety, but rather how effective the jab is.
The questions centre around efficacy levels.
Three were reported from the trial - an overall efficacy of 70%, a lower one of 62% and a high of 90%.
That's because different doses of the vaccine were mistakenly used in the trial. Some volunteers were given shots half the planned strength, in error. Yet that "wrong" dose turned out to be a winner.
What does that mean?
Some of the shots were weaker than they were designed to be, containing much less of the ingredient that is meant to give a person immunity.
The jab is actually two shots, with the second given a month after the first as a booster.
While most of the volunteers in the trial got the correct dose for both of their two shots, some didn't.
Regulators were told about the error early on and they agreed that the trial could continue and more volunteers could be immunised.
The error had no effect on vaccine safety.
What were the results?
About 3,000 participants were given the half dose and then a full dose four weeks later, and this regime appeared to provide the most protection or efficacy in the trial - around 90%.
In the larger group of nearly 9,000 volunteers, who were given two full doses also four weeks apart, efficacy was 62%.
AstraZeneca reported these percentages and also said that its vaccine was, on average, 70% effective at preventing Covid-19 illness. The figures left some experts scratching their head.
Prof David Salisbury, immunisation expert and associate fellow of the global health program at the Chatham House think tank, said: "You've taken two studies for which different doses were used and come up with a composite that doesn't represent either of the doses. I think many people are having trouble with that.″
AstraZeneca stressed that the data are preliminary, rather than full and final - which is true for the reported Pfizer and Moderna jab results too. It is science by press release.
When they can, all of the companies will publish full results in medical journals for public scrutiny.
And they are submitting full data to regulators to apply for emergency approval so that countries can start using these three different vaccines to immunise whole populations.
Does it change anything?
The US regulator, called the FDA, have said any Covid vaccine needs to be at least 50% effective to be useful in fighting the pandemic.
Even if you take the lowest figure of effectiveness for the AstraZeneca jab, it still passes that benchmark.
The efficacy analysis was based on 131 cases of Covid-19 that occurred in the study participants:
One idea is that a low then high dose shot may be a better mimic of a coronavirus infection and lead to a better immune response.
But it is possible that the volunteers who got the half doses are somewhat different to those who got two big shots.
Moncef Slaoui, the scientific head of the US's Operation Warp Speed - the programme to supply America with vaccines - told US reporters that the half-dose group only included people younger than 55.
Since age is the biggest risk factor for getting seriously ill with Covid-19, a vaccine that protects the elderly is extremely important.
However, results from an earlier phase two study of the Oxford vaccine, published in The Lancet medical journal, showed the vaccine produced a strong response in all age groups.
AstraZeneca said: "The studies were conducted to the highest standards.
"More data will continue to accumulate and additional analysis will be conducted refining the efficacy reading and establishing the duration of protection."
The company said it would run a new study to evaluate a lower dosage that performed better.
Its chief executive Pascal Soriot said it would probably be another "international study, but this one could be faster because we know the efficacy is high so we need a smaller number of patients".
What do other experts say?
Although the dosing was different, the rest of the study didn't change from the original plan.
Prof Peter Openshaw, an expert at Imperial College London, says the take home message should be that we have three very promising Covid vaccines that could soon become available to help save lives.
"We have to wait for the full data and to see how the regulators view the results.
"All we have to go on is a limited data release. The protection from the Oxford AstraZeneca vaccine may be less than that from the mRNA vaccines, but we need to wait and see.
"It is remarkable that each of the trials that are now reporting shows protection, which we did not know was going to be possible."
He added: "We have been wanting vaccines for many diseases for a long time and they haven't arrived - HIV, TB and malaria being good examples.
"The results so far seem to show that it can be done for Covid-19, and that's very good news indeed."
https://www.bbc.co.uk/news/health-55086927
vaccine dose error
On Monday, the world heard how the UK's Covid vaccine - from AstraZeneca and Oxford University - was highly effective in advanced trials
It gave hope of another new jab to fight the pandemic that should be cheaper and easier to distribute than the Pfizer/BioNTech and Moderna mRNA vaccines that announced similarly impressive results just days before.
But after the jubilation, some negative press has followed.
On Thursday, multiple news outlets in the UK and US reported that there were questions over the data. They weren't about safety, but rather how effective the jab is.
The questions centre around efficacy levels.
Three were reported from the trial - an overall efficacy of 70%, a lower one of 62% and a high of 90%.
That's because different doses of the vaccine were mistakenly used in the trial. Some volunteers were given shots half the planned strength, in error. Yet that "wrong" dose turned out to be a winner.
What does that mean?
Some of the shots were weaker than they were designed to be, containing much less of the ingredient that is meant to give a person immunity.
The jab is actually two shots, with the second given a month after the first as a booster.
While most of the volunteers in the trial got the correct dose for both of their two shots, some didn't.
Regulators were told about the error early on and they agreed that the trial could continue and more volunteers could be immunised.
The error had no effect on vaccine safety.
What were the results?
About 3,000 participants were given the half dose and then a full dose four weeks later, and this regime appeared to provide the most protection or efficacy in the trial - around 90%.
In the larger group of nearly 9,000 volunteers, who were given two full doses also four weeks apart, efficacy was 62%.
AstraZeneca reported these percentages and also said that its vaccine was, on average, 70% effective at preventing Covid-19 illness. The figures left some experts scratching their head.
Prof David Salisbury, immunisation expert and associate fellow of the global health program at the Chatham House think tank, said: "You've taken two studies for which different doses were used and come up with a composite that doesn't represent either of the doses. I think many people are having trouble with that.″
AstraZeneca stressed that the data are preliminary, rather than full and final - which is true for the reported Pfizer and Moderna jab results too. It is science by press release.
When they can, all of the companies will publish full results in medical journals for public scrutiny.
And they are submitting full data to regulators to apply for emergency approval so that countries can start using these three different vaccines to immunise whole populations.
Does it change anything?
The US regulator, called the FDA, have said any Covid vaccine needs to be at least 50% effective to be useful in fighting the pandemic.
Even if you take the lowest figure of effectiveness for the AstraZeneca jab, it still passes that benchmark.
The efficacy analysis was based on 131 cases of Covid-19 that occurred in the study participants:
- 101 of these cases happened in people who received dummy injections (either a saline jab or a meningitis vaccine).
The other 30 were in people who received the real jab - three who got the half-strength initial dose and 27 who had the two full doses.
One idea is that a low then high dose shot may be a better mimic of a coronavirus infection and lead to a better immune response.
But it is possible that the volunteers who got the half doses are somewhat different to those who got two big shots.
Moncef Slaoui, the scientific head of the US's Operation Warp Speed - the programme to supply America with vaccines - told US reporters that the half-dose group only included people younger than 55.
Since age is the biggest risk factor for getting seriously ill with Covid-19, a vaccine that protects the elderly is extremely important.
However, results from an earlier phase two study of the Oxford vaccine, published in The Lancet medical journal, showed the vaccine produced a strong response in all age groups.
AstraZeneca said: "The studies were conducted to the highest standards.
"More data will continue to accumulate and additional analysis will be conducted refining the efficacy reading and establishing the duration of protection."
The company said it would run a new study to evaluate a lower dosage that performed better.
Its chief executive Pascal Soriot said it would probably be another "international study, but this one could be faster because we know the efficacy is high so we need a smaller number of patients".
What do other experts say?
Although the dosing was different, the rest of the study didn't change from the original plan.
Prof Peter Openshaw, an expert at Imperial College London, says the take home message should be that we have three very promising Covid vaccines that could soon become available to help save lives.
"We have to wait for the full data and to see how the regulators view the results.
"All we have to go on is a limited data release. The protection from the Oxford AstraZeneca vaccine may be less than that from the mRNA vaccines, but we need to wait and see.
"It is remarkable that each of the trials that are now reporting shows protection, which we did not know was going to be possible."
He added: "We have been wanting vaccines for many diseases for a long time and they haven't arrived - HIV, TB and malaria being good examples.
"The results so far seem to show that it can be done for Covid-19, and that's very good news indeed."
https://www.bbc.co.uk/news/health-55086927
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
End of the Pandemic
Is Now in Sight
A year of scientific uncertainty is over. Two vaccines look like they will work, and more should follow
For all that scientists have done to tame the biological world, there are still things that lie outside the realm of human knowledge. The coronavirus was one such alarming reminder, when it emerged with murky origins in late 2019 and found naive, unwitting hosts in the human body.
Even as science began to unravel many of the virus’s mysteries—how it spreads, how it tricks its way into cells, how it kills—a fundamental unknown about vaccines hung over the pandemic and our collective human fate: Vaccines can stop many, but not all, viruses. Could they stop this one?
The answer, we now know, is yes. A resounding yes. Pfizer and Moderna have separately released preliminary data that suggest their vaccines are both more than 90 percent effective, far more than many scientists expected.
Neither company has publicly shared the full scope of their data, but independent clinical-trial monitoring boards have reviewed the results, and the FDA will soon scrutinize the vaccines for emergency use authorization. Unless the data take an unexpected turn, initial doses should be available in December.
The tasks that lie ahead—manufacturing vaccines at scale, distributing them via a cold or even ultracold chain, and persuading wary Americans to take them—are not trivial, but they are all within the realm of human knowledge.
The most tenuous moment is over: The scientific uncertainty at the heart of COVID-19 vaccines is resolved. Vaccines work. And for that, we can breathe a collective sigh of relief. “It makes it now clear that vaccines will be our way out of this pandemic,” says Kanta Subbarao, a virologist at the Doherty Institute, who has studied emerging viruses.
The invention of vaccines against a virus identified only 10 months ago is an extraordinary scientific achievement. They are the fastest vaccines ever developed, by a margin of years. From virtually the day Chinese scientists shared the genetic sequence of a new coronavirus in January, researchers began designing vaccines that might train the immune system to recognize the still-unnamed virus. They needed to identify a suitable piece of the virus to turn into a vaccine, and one promising target was the spike-shaped proteins that decorate the new virus’s outer shell.
Pfizer and Moderna’s vaccines both rely on the spike protein, as do many vaccine candidates still in development. These initial successes suggest this strategy works; several more COVID-19 vaccines may soon cross the finish line. To vaccinate billions of people across the globe and bring the pandemic to a timely end, we will need all the vaccines we can get.
But it is no accident or surprise that Moderna and Pfizer are first out of the gate. They both bet on a new and hitherto unproven idea of using mRNA, which has the long-promised advantage of speed. This idea has now survived a trial by pandemic and emerged likely triumphant. If mRNA vaccines help end the pandemic and restore normal life, they may also usher in a new era for vaccine development.
The human immune system is awesome in its power, but an untrained one does not know how to aim its fire. That’s where vaccines come in. They present a harmless snapshot of a pathogen, a “wanted” poster, if you will, that primes the immune system to recognize the real virus when it comes along.
Traditionally, this snapshot could be in the form of a weakened virus or an inactivated virus or a particularly distinctive viral molecule. But those approaches require vaccine makers to manufacture viruses and their molecules, which takes time and expertise. Both are lacking during a pandemic caused by a novel virus.
mRNA vaccines offer a clever shortcut. We humans don’t need to intellectually work out how to make viruses; our bodies are already very, very good at incubating them. When the coronavirus infects us, it hijacks our cellular machinery, turning our cells into miniature factories that churn out infectious viruses.
The mRNA vaccine makes this vulnerability into a strength. What if we can trick our own cells into making just one individually harmless, though very recognizable, viral protein? The coronavirus’s spike protein fits this description, and the instructions for making it can be encoded into genetic material called mRNA.
Both vaccines, from Moderna and from Pfizer’s collaboration with the smaller German company BioNTech, package slightly modified spike-protein mRNA inside a tiny protective bubble of fat. Human cells take up this bubble and simply follow the directions to make spike protein. The cells then display these spike proteins, presenting them as strange baubles to the immune system. Recognizing these viral proteins as foreign, the immune system begins building an arsenal to prepare for the moment a virus bearing this spike protein appears.
This overall process mimics the steps of infection better than some traditional vaccines, which suggests that mRNA vaccines may provoke a better immune response for certain diseases. When you inject vaccines made of inactivated viruses or viral pieces, they can’t get inside the cell, and the cell can’t present those viral pieces to the immune system.
Those vaccines can still elicit proteins called antibodies, which neutralize the virus, but they have a harder time stimulating T cells, which make up another important part of the immune response. (Weakened viruses used in vaccines can get inside cells, but risk causing an actual infection if something goes awry. mRNA vaccines cannot cause infection because they do not contain the whole virus.)
Moreover, inactivated viruses or viral pieces tend to disappear from the body within a day, but mRNA vaccines can continue to produce spike protein for two weeks, says Drew Weissman, an immunologist at the University of Pennsylvania, whose mRNA vaccine research has been licensed by both BioNTech and Moderna. The longer the spike protein is around, the better for an immune response.
All of this is how mRNA vaccines should work in theory. But no one on Earth, until last week, knew whether mRNA vaccines actually do work in humans for COVID-19. Although scientists had prototyped other mRNA vaccines before the pandemic, the technology was still new.
None had been put through the paces of a large clinical trial. And the human immune system is notoriously complicated and unpredictable. Immunology is, as my colleague Ed Yong has written, where intuition goes to die. Vaccines can even make diseases more severe, rather than less.
The data from these large clinical trials from Pfizer/BioNTech and Moderna are the first, real-world proof that mRNA vaccines protect against disease as expected. The hope, in the many years when mRNA vaccine research flew under the radar, was that the technology would deliver results quickly in a pandemic. And now it has.
“What a relief,” says Barney Graham, a virologist at the National Institutes of Health, who helped design the spike protein for the Moderna vaccine. “You can make thousands of decisions, and thousands of things have to go right for this to actually come out and work. You’re just worried that you have made some wrong turns along the way.”
For Graham, this vaccine is a culmination of years of such decisions, long predating the discovery of the coronavirus that causes COVID-19. He and his collaborators had homed in on the importance of spike protein in another virus, called respiratory syncytial virus, and figured out how to make the protein more stable and thus suitable for vaccines. This modification appears in both Pfizer/BioNTech’s and Moderna’s vaccines, as well as other leading vaccine candidates.
The spectacular efficacy of these vaccines, should the preliminary data hold, likely also has to do with the choice of spike protein as vaccine target. On one hand, scientists were prepared for the spike protein, thanks to research like Graham’s. On the other hand, the coronavirus’s spike protein offered an opening. Three separate components of the immune system—antibodies, helper cells, and killer T cells—all respond to the spike protein, which isn’t the case with most viruses.
In this, we were lucky. “It’s the three punches,” says Alessandro Sette. Working with Shane Crotty, his fellow immunologist at the La Jolla Institute, Sette found that COVID-19 patients whose immune systems can marshal all three responses against the spike protein tend to fare the best.
The fact that most people can recover from COVID-19 was always encouraging news; it meant a vaccine simply needed to jump-start the immune system, which could then take on the virus itself. But no definitive piece of evidence existed that proved COVID-19 vaccines would be a slam dunk. “There’s nothing like a Phase 3 clinical trial,” Crotty says. “You don’t know what’s gonna happen with a vaccine until it happens, because the virus is complicated and the immune system is complicated.”
Experts anticipate that the ongoing trials will clarify still-unanswered questions about the COVID-19 vaccines. For example, Ruth Karron, the director of the Center for Immunization Research at Johns Hopkins University, asks, does the vaccine prevent only a patient’s symptoms? Or does it keep them from spreading the virus?
How long will immunity last? How well does it protect the elderly, many of whom have a weaker response to the flu vaccine? So far, Pfizer has noted that its vaccine seems to protect the elderly just as well, which is good news because they are especially vulnerable to COVID-19.
Several more vaccines using the spike protein are in clinical trials too. They rely on a suite of different vaccine technologies, including weakened viruses, inactivated viruses, viral proteins, and another fairly new concept called DNA vaccines. Never before have companies tested so many different types of vaccines against the same virus, which might end up revealing something new about vaccines in general.
You now have the same spike protein delivered in many different ways, Sette points out. How will the vaccines behave differently? Will they each stimulate different parts of the immune system? And which parts are best for protecting against the coronavirus? The pandemic is an opportunity to compare different types of vaccines head-on.
If the two mRNA vaccines continue to be as good as they initially seem, their success will likely crack open a whole new world of mRNA vaccines. Scientists are already testing them against currently un-vaccinable viruses such as Zika and cytomegalovirus and trying to make improved versions of existing vaccines, such as for the flu. Another possibility lies in personalized mRNA vaccines that can stimulate the immune system to fight cancer.
But the next few months will be a test of one potential downside of mRNA vaccines: their extreme fragility. mRNA is an inherently unstable molecule, which is why it needs that protective bubble of fat, called a lipid nanoparticle. But the lipid nanoparticle itself is exquisitely sensitive to temperature.
For longer-term storage, Pfizer/BioNTech’s vaccine has to be stored at –70 degrees Celsius and Moderna’s at –20 Celsius, though they can be kept at higher temperatures for a shorter amount of time. Pfizer/BioNTech and Moderna have said they can collectively supply enough doses for 22.5 million people in the United States by the end of the year.
Distributing the limited vaccines fairly and smoothly will be a massive political and logistical challenge, especially as it begins during a bitter transition of power in Washington. The vaccine is a scientific triumph, but the past eight months have made clear how much pandemic preparedness is not only about scientific research.
Ensuring adequate supplies of tests and personal protective equipment, providing economic relief, and communicating the known risks of COVID-19 transmission are all well within the realm of human knowledge, yet the U.S. government has failed at all of that.
The vaccine by itself cannot slow the dangerous trajectory of COVID-19 hospitalizations this fall or save the many people who may die by Christmas. But it can give us hope that the pandemic will end. Every infection we prevent now—through masking and social distancing—is an infection that can, eventually, be prevented forever through vaccines.
https://www.theatlantic.com/health/arch ... obal-en-GB
Is Now in Sight
A year of scientific uncertainty is over. Two vaccines look like they will work, and more should follow
For all that scientists have done to tame the biological world, there are still things that lie outside the realm of human knowledge. The coronavirus was one such alarming reminder, when it emerged with murky origins in late 2019 and found naive, unwitting hosts in the human body.
Even as science began to unravel many of the virus’s mysteries—how it spreads, how it tricks its way into cells, how it kills—a fundamental unknown about vaccines hung over the pandemic and our collective human fate: Vaccines can stop many, but not all, viruses. Could they stop this one?
The answer, we now know, is yes. A resounding yes. Pfizer and Moderna have separately released preliminary data that suggest their vaccines are both more than 90 percent effective, far more than many scientists expected.
Neither company has publicly shared the full scope of their data, but independent clinical-trial monitoring boards have reviewed the results, and the FDA will soon scrutinize the vaccines for emergency use authorization. Unless the data take an unexpected turn, initial doses should be available in December.
The tasks that lie ahead—manufacturing vaccines at scale, distributing them via a cold or even ultracold chain, and persuading wary Americans to take them—are not trivial, but they are all within the realm of human knowledge.
The most tenuous moment is over: The scientific uncertainty at the heart of COVID-19 vaccines is resolved. Vaccines work. And for that, we can breathe a collective sigh of relief. “It makes it now clear that vaccines will be our way out of this pandemic,” says Kanta Subbarao, a virologist at the Doherty Institute, who has studied emerging viruses.
The invention of vaccines against a virus identified only 10 months ago is an extraordinary scientific achievement. They are the fastest vaccines ever developed, by a margin of years. From virtually the day Chinese scientists shared the genetic sequence of a new coronavirus in January, researchers began designing vaccines that might train the immune system to recognize the still-unnamed virus. They needed to identify a suitable piece of the virus to turn into a vaccine, and one promising target was the spike-shaped proteins that decorate the new virus’s outer shell.
Pfizer and Moderna’s vaccines both rely on the spike protein, as do many vaccine candidates still in development. These initial successes suggest this strategy works; several more COVID-19 vaccines may soon cross the finish line. To vaccinate billions of people across the globe and bring the pandemic to a timely end, we will need all the vaccines we can get.
But it is no accident or surprise that Moderna and Pfizer are first out of the gate. They both bet on a new and hitherto unproven idea of using mRNA, which has the long-promised advantage of speed. This idea has now survived a trial by pandemic and emerged likely triumphant. If mRNA vaccines help end the pandemic and restore normal life, they may also usher in a new era for vaccine development.
The human immune system is awesome in its power, but an untrained one does not know how to aim its fire. That’s where vaccines come in. They present a harmless snapshot of a pathogen, a “wanted” poster, if you will, that primes the immune system to recognize the real virus when it comes along.
Traditionally, this snapshot could be in the form of a weakened virus or an inactivated virus or a particularly distinctive viral molecule. But those approaches require vaccine makers to manufacture viruses and their molecules, which takes time and expertise. Both are lacking during a pandemic caused by a novel virus.
mRNA vaccines offer a clever shortcut. We humans don’t need to intellectually work out how to make viruses; our bodies are already very, very good at incubating them. When the coronavirus infects us, it hijacks our cellular machinery, turning our cells into miniature factories that churn out infectious viruses.
The mRNA vaccine makes this vulnerability into a strength. What if we can trick our own cells into making just one individually harmless, though very recognizable, viral protein? The coronavirus’s spike protein fits this description, and the instructions for making it can be encoded into genetic material called mRNA.
Both vaccines, from Moderna and from Pfizer’s collaboration with the smaller German company BioNTech, package slightly modified spike-protein mRNA inside a tiny protective bubble of fat. Human cells take up this bubble and simply follow the directions to make spike protein. The cells then display these spike proteins, presenting them as strange baubles to the immune system. Recognizing these viral proteins as foreign, the immune system begins building an arsenal to prepare for the moment a virus bearing this spike protein appears.
This overall process mimics the steps of infection better than some traditional vaccines, which suggests that mRNA vaccines may provoke a better immune response for certain diseases. When you inject vaccines made of inactivated viruses or viral pieces, they can’t get inside the cell, and the cell can’t present those viral pieces to the immune system.
Those vaccines can still elicit proteins called antibodies, which neutralize the virus, but they have a harder time stimulating T cells, which make up another important part of the immune response. (Weakened viruses used in vaccines can get inside cells, but risk causing an actual infection if something goes awry. mRNA vaccines cannot cause infection because they do not contain the whole virus.)
Moreover, inactivated viruses or viral pieces tend to disappear from the body within a day, but mRNA vaccines can continue to produce spike protein for two weeks, says Drew Weissman, an immunologist at the University of Pennsylvania, whose mRNA vaccine research has been licensed by both BioNTech and Moderna. The longer the spike protein is around, the better for an immune response.
All of this is how mRNA vaccines should work in theory. But no one on Earth, until last week, knew whether mRNA vaccines actually do work in humans for COVID-19. Although scientists had prototyped other mRNA vaccines before the pandemic, the technology was still new.
None had been put through the paces of a large clinical trial. And the human immune system is notoriously complicated and unpredictable. Immunology is, as my colleague Ed Yong has written, where intuition goes to die. Vaccines can even make diseases more severe, rather than less.
The data from these large clinical trials from Pfizer/BioNTech and Moderna are the first, real-world proof that mRNA vaccines protect against disease as expected. The hope, in the many years when mRNA vaccine research flew under the radar, was that the technology would deliver results quickly in a pandemic. And now it has.
“What a relief,” says Barney Graham, a virologist at the National Institutes of Health, who helped design the spike protein for the Moderna vaccine. “You can make thousands of decisions, and thousands of things have to go right for this to actually come out and work. You’re just worried that you have made some wrong turns along the way.”
For Graham, this vaccine is a culmination of years of such decisions, long predating the discovery of the coronavirus that causes COVID-19. He and his collaborators had homed in on the importance of spike protein in another virus, called respiratory syncytial virus, and figured out how to make the protein more stable and thus suitable for vaccines. This modification appears in both Pfizer/BioNTech’s and Moderna’s vaccines, as well as other leading vaccine candidates.
The spectacular efficacy of these vaccines, should the preliminary data hold, likely also has to do with the choice of spike protein as vaccine target. On one hand, scientists were prepared for the spike protein, thanks to research like Graham’s. On the other hand, the coronavirus’s spike protein offered an opening. Three separate components of the immune system—antibodies, helper cells, and killer T cells—all respond to the spike protein, which isn’t the case with most viruses.
In this, we were lucky. “It’s the three punches,” says Alessandro Sette. Working with Shane Crotty, his fellow immunologist at the La Jolla Institute, Sette found that COVID-19 patients whose immune systems can marshal all three responses against the spike protein tend to fare the best.
The fact that most people can recover from COVID-19 was always encouraging news; it meant a vaccine simply needed to jump-start the immune system, which could then take on the virus itself. But no definitive piece of evidence existed that proved COVID-19 vaccines would be a slam dunk. “There’s nothing like a Phase 3 clinical trial,” Crotty says. “You don’t know what’s gonna happen with a vaccine until it happens, because the virus is complicated and the immune system is complicated.”
Experts anticipate that the ongoing trials will clarify still-unanswered questions about the COVID-19 vaccines. For example, Ruth Karron, the director of the Center for Immunization Research at Johns Hopkins University, asks, does the vaccine prevent only a patient’s symptoms? Or does it keep them from spreading the virus?
How long will immunity last? How well does it protect the elderly, many of whom have a weaker response to the flu vaccine? So far, Pfizer has noted that its vaccine seems to protect the elderly just as well, which is good news because they are especially vulnerable to COVID-19.
Several more vaccines using the spike protein are in clinical trials too. They rely on a suite of different vaccine technologies, including weakened viruses, inactivated viruses, viral proteins, and another fairly new concept called DNA vaccines. Never before have companies tested so many different types of vaccines against the same virus, which might end up revealing something new about vaccines in general.
You now have the same spike protein delivered in many different ways, Sette points out. How will the vaccines behave differently? Will they each stimulate different parts of the immune system? And which parts are best for protecting against the coronavirus? The pandemic is an opportunity to compare different types of vaccines head-on.
If the two mRNA vaccines continue to be as good as they initially seem, their success will likely crack open a whole new world of mRNA vaccines. Scientists are already testing them against currently un-vaccinable viruses such as Zika and cytomegalovirus and trying to make improved versions of existing vaccines, such as for the flu. Another possibility lies in personalized mRNA vaccines that can stimulate the immune system to fight cancer.
But the next few months will be a test of one potential downside of mRNA vaccines: their extreme fragility. mRNA is an inherently unstable molecule, which is why it needs that protective bubble of fat, called a lipid nanoparticle. But the lipid nanoparticle itself is exquisitely sensitive to temperature.
For longer-term storage, Pfizer/BioNTech’s vaccine has to be stored at –70 degrees Celsius and Moderna’s at –20 Celsius, though they can be kept at higher temperatures for a shorter amount of time. Pfizer/BioNTech and Moderna have said they can collectively supply enough doses for 22.5 million people in the United States by the end of the year.
Distributing the limited vaccines fairly and smoothly will be a massive political and logistical challenge, especially as it begins during a bitter transition of power in Washington. The vaccine is a scientific triumph, but the past eight months have made clear how much pandemic preparedness is not only about scientific research.
Ensuring adequate supplies of tests and personal protective equipment, providing economic relief, and communicating the known risks of COVID-19 transmission are all well within the realm of human knowledge, yet the U.S. government has failed at all of that.
The vaccine by itself cannot slow the dangerous trajectory of COVID-19 hospitalizations this fall or save the many people who may die by Christmas. But it can give us hope that the pandemic will end. Every infection we prevent now—through masking and social distancing—is an infection that can, eventually, be prevented forever through vaccines.
https://www.theatlantic.com/health/arch ... obal-en-GB
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Are Covid-19 Vaccines 95% Effective?
You might assume that 95 out of every 100 people vaccinated will be protected from Covid-19. But that’s not how the math works
Experts say it’s easy to misconstrue early results because the language vaccine researchers use to talk about their trials can be hard for outsiders to understand.
The front-runners in the vaccine race seem to be working far better than anyone expected: Pfizer and BioNTech announced this week that their vaccine had an efficacy rate of 95 percent. Moderna put the figure for its vaccine at 94.5 percent. In Russia, the makers of the Sputnik vaccine claimed their efficacy rate was over 90 percent.
“These are game changers,” said Dr. Gregory Poland, a vaccine researcher at the Mayo Clinic. “We were all expecting 50 to 70 percent.” Indeed, the Food and Drug Administration had said it would consider granting emergency approval for vaccines that showed just 50 percent efficacy.
From the headlines, you might well assume that these vaccines — which some people may receive in a matter of weeks — will protect 95 out of 100 people who get them. But that’s not actually what the trials have shown. Exactly how the vaccines perform out in the real world will depend on a lot of factors we just don’t have answers to yet — such as whether vaccinated people can get asymptomatic infections and how many people will get vaccinated.
Here’s what you need to know about the actual effectiveness of these vaccines.
What do the companies mean when they say their vaccines are 95 percent effective?
The fundamental logic behind today’s vaccine trials was worked out by statisticians over a century ago. Researchers vaccinate some people and give a placebo to others. They then wait for participants to get sick and look at how many of the illnesses came from each group.
A 95 percent efficacy is certainly compelling evidence that a vaccine works well. But that number doesn’t tell you what your chances are of becoming sick if you get vaccinated. And on its own, it also doesn’t say how well the vaccine will bring down Covid-19 across the United States.
What’s the difference between efficacy and effectiveness?
Efficacy and effectiveness are related to each other, but they’re not the same thing. And vaccine experts say it’s crucial not to mix them up. Efficacy is just a measurement made during a clinical trial. “Effectiveness is how well the vaccine works out in the real world,” said Naor Bar-Zeev, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health.
It’s possible that the effectiveness of coronavirus vaccines will match their impressive efficacy in clinical trials. But if previous vaccines are any guide, effectiveness may prove somewhat lower.
The mismatch comes about because the people who join clinical trials are not a perfect reflection of the population at large. Out in the real world, people may have a host of chronic health problems that could interfere with a vaccine’s protection, for example.
The Centers for Disease Control and Prevention has a long history of following the effectiveness of vaccines after they’re approved. On Thursday, the agency posted information on its website about its plans to study the effectiveness of coronavirus vaccines. It will find opportunities to compare the health of vaccinated people to others in their communities who have not received a vaccine.
What exactly are these vaccines effective at doing?
The clinical trials run by Pfizer and other companies were specifically designed to see whether vaccines protect people from getting sick from Covid-19. If volunteers developed symptoms like a fever or cough, they were then tested for the coronavirus.
But there’s abundant evidence that people can get infected with the coronavirus without ever showing symptoms. And so it’s possible that a number of people who got vaccinated in the clinical trials got infected, too, without ever realizing it. If those cases indeed exist, none of them are reflected in the 95 percent efficacy rate.
People who are asymptomatic can still spread the virus to others. Some studies suggest that they produce fewer viruses, making them less of a threat than infected people who go on to develop symptoms. But if people get vaccinated and then stop wearing masks and taking other safety measures, their chances of spreading the coronavirus to others could increase.
“You could get this paradoxical situation of things getting worse,” said Dr. Bar-Zeev.
Will these vaccines put a dent in the epidemic?
Vaccines don’t protect only the people who get them. Because they slow the spread of the virus, they can, over time, also drive down new infection rates and protect society as a whole.
Scientists call this broad form of effectiveness a vaccine’s impact. The smallpox vaccine had the greatest impact of all, driving the virus into oblivion in the 1970s. But even a vaccine with extremely high efficacy in clinical trials will have a small impact if only a few people end up getting it.
“Vaccines don’t save lives,” said A. David Paltiel, a professor at the Yale School of Public Health. “Vaccination programs save lives.”
On Thursday, Dr. Paltiel and his colleagues published a study in the journal Health Affairs in which they simulated the coming rollout of coronavirus vaccines. They modeled vaccines with efficacy rates ranging from high to low, but also considered how quickly and widely a vaccine could be distributed as the pandemic continues to rage.
The results, Dr. Paltiel said, were heartbreaking. He and his colleagues found that when it comes to cutting down on infections, hospitalizations and deaths, the deployment mattered just as much as the efficacy. The study left Dr. Paltiel worried that the United States has not done enough to prepare for the massive distribution of the vaccine in the months to come.
“Time is really running out,” he warned. “Infrastructure is going to contribute at least as much, if not more, than the vaccine itself to the success of the program.”
https://www.nytimes.com/2020/11/20/heal ... obal-en-GB
You might assume that 95 out of every 100 people vaccinated will be protected from Covid-19. But that’s not how the math works
Experts say it’s easy to misconstrue early results because the language vaccine researchers use to talk about their trials can be hard for outsiders to understand.
The front-runners in the vaccine race seem to be working far better than anyone expected: Pfizer and BioNTech announced this week that their vaccine had an efficacy rate of 95 percent. Moderna put the figure for its vaccine at 94.5 percent. In Russia, the makers of the Sputnik vaccine claimed their efficacy rate was over 90 percent.
“These are game changers,” said Dr. Gregory Poland, a vaccine researcher at the Mayo Clinic. “We were all expecting 50 to 70 percent.” Indeed, the Food and Drug Administration had said it would consider granting emergency approval for vaccines that showed just 50 percent efficacy.
From the headlines, you might well assume that these vaccines — which some people may receive in a matter of weeks — will protect 95 out of 100 people who get them. But that’s not actually what the trials have shown. Exactly how the vaccines perform out in the real world will depend on a lot of factors we just don’t have answers to yet — such as whether vaccinated people can get asymptomatic infections and how many people will get vaccinated.
Here’s what you need to know about the actual effectiveness of these vaccines.
What do the companies mean when they say their vaccines are 95 percent effective?
The fundamental logic behind today’s vaccine trials was worked out by statisticians over a century ago. Researchers vaccinate some people and give a placebo to others. They then wait for participants to get sick and look at how many of the illnesses came from each group.
- In the case of Pfizer, for example, the company recruited 43,661 volunteers and waited for 170 people to come down with symptoms of Covid-19 and then get a positive test. Out of these 170, 162 had received a placebo shot, and just eight had received the real vaccine
A 95 percent efficacy is certainly compelling evidence that a vaccine works well. But that number doesn’t tell you what your chances are of becoming sick if you get vaccinated. And on its own, it also doesn’t say how well the vaccine will bring down Covid-19 across the United States.
What’s the difference between efficacy and effectiveness?
Efficacy and effectiveness are related to each other, but they’re not the same thing. And vaccine experts say it’s crucial not to mix them up. Efficacy is just a measurement made during a clinical trial. “Effectiveness is how well the vaccine works out in the real world,” said Naor Bar-Zeev, an epidemiologist at the Johns Hopkins Bloomberg School of Public Health.
It’s possible that the effectiveness of coronavirus vaccines will match their impressive efficacy in clinical trials. But if previous vaccines are any guide, effectiveness may prove somewhat lower.
The mismatch comes about because the people who join clinical trials are not a perfect reflection of the population at large. Out in the real world, people may have a host of chronic health problems that could interfere with a vaccine’s protection, for example.
The Centers for Disease Control and Prevention has a long history of following the effectiveness of vaccines after they’re approved. On Thursday, the agency posted information on its website about its plans to study the effectiveness of coronavirus vaccines. It will find opportunities to compare the health of vaccinated people to others in their communities who have not received a vaccine.
What exactly are these vaccines effective at doing?
The clinical trials run by Pfizer and other companies were specifically designed to see whether vaccines protect people from getting sick from Covid-19. If volunteers developed symptoms like a fever or cough, they were then tested for the coronavirus.
But there’s abundant evidence that people can get infected with the coronavirus without ever showing symptoms. And so it’s possible that a number of people who got vaccinated in the clinical trials got infected, too, without ever realizing it. If those cases indeed exist, none of them are reflected in the 95 percent efficacy rate.
People who are asymptomatic can still spread the virus to others. Some studies suggest that they produce fewer viruses, making them less of a threat than infected people who go on to develop symptoms. But if people get vaccinated and then stop wearing masks and taking other safety measures, their chances of spreading the coronavirus to others could increase.
“You could get this paradoxical situation of things getting worse,” said Dr. Bar-Zeev.
Will these vaccines put a dent in the epidemic?
Vaccines don’t protect only the people who get them. Because they slow the spread of the virus, they can, over time, also drive down new infection rates and protect society as a whole.
Scientists call this broad form of effectiveness a vaccine’s impact. The smallpox vaccine had the greatest impact of all, driving the virus into oblivion in the 1970s. But even a vaccine with extremely high efficacy in clinical trials will have a small impact if only a few people end up getting it.
“Vaccines don’t save lives,” said A. David Paltiel, a professor at the Yale School of Public Health. “Vaccination programs save lives.”
On Thursday, Dr. Paltiel and his colleagues published a study in the journal Health Affairs in which they simulated the coming rollout of coronavirus vaccines. They modeled vaccines with efficacy rates ranging from high to low, but also considered how quickly and widely a vaccine could be distributed as the pandemic continues to rage.
The results, Dr. Paltiel said, were heartbreaking. He and his colleagues found that when it comes to cutting down on infections, hospitalizations and deaths, the deployment mattered just as much as the efficacy. The study left Dr. Paltiel worried that the United States has not done enough to prepare for the massive distribution of the vaccine in the months to come.
“Time is really running out,” he warned. “Infrastructure is going to contribute at least as much, if not more, than the vaccine itself to the success of the program.”
https://www.nytimes.com/2020/11/20/heal ... obal-en-GB
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Covid-19: Pfizer/BioNTech vaccine
safe for use in UK from next week
The UK has become the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine, paving the way for mass vaccination
The first doses are already on their way to the UK, with 800,000 due in the coming days, Pfizer said.
Health Secretary Matt Hancock said the NHS will contact people about jabs.
Elderly people in care homes and care home staff have been placed top of the priority list, followed by over-80s and health and care staff.
But because hospitals already have the facilities to store the vaccine at -70C, as required, the very first vaccinations are likely to take place there - for care home staff, NHS staff and patients - so none of the vaccine is wasted.
The Pfizer/BioNTech jab is the fastest vaccine to go from concept to reality, taking only 10 months to follow the same steps that normally span 10 years.
The UK has already ordered 40 million doses of the jab - enough to vaccinate 20 million people.
The doses will be rolled out as quickly as they can be made by Pfizer in Belgium, Mr Hancock said, with the first load next week and then "several millions" throughout December.
Scottish First Minister Nicola Sturgeon said the first people in Scotland will be immunised on Tuesday.
The bulk of the rollout will be next year, Mr Hancock said. "2020 has been just awful and 2021 is going to be better," he said.
"I'm confident now, with the news today, that from spring, from Easter onwards, things are going to be better. And we're going to have a summer next year that everybody can enjoy."
Prime Minister Boris Johnson added: "It's the protection of vaccines that will ultimately allow us to reclaim our lives and get the economy moving again."
media captionHeath Secretary Matt Hancock says he is "thrilled" that the vaccine has been approved
The free vaccine will not be compulsory and there will be three ways of vaccinating people across the UK:
It is thought the vaccination network could start delivering more than one million doses a week once enough doses are available.
NHS chief executive Sir Simon Stevens said the health service was preparing for "the largest-scale vaccination campaign in our country's history".
But experts said people still need to remain vigilant and follow rules to stop the virus spreading - including with social distancing, face masks and self-isolation.
"We can't lower our guard yet," said the government's chief medical adviser Prof Chris Whitty.
This is the news we have all been waiting for
Analysis box by Nick Triggle, health correspondent
This is the news we have all been waiting for.
The NHS has already been gearing up for this moment for some time. The venues are in place and there is provision for extra staffing, with even lifeguards and airline staff to be brought in to help with the effort.
But the biggest hurdle will be supply.
The UK has been promised 40 million doses by the spring - enough to give the required two jabs to health and care workers and everyone over 65. But in the first few weeks of winter, our ability to vaccinate could easily outstrip supply.
Ministers say they will have 800,000 doses in the country within days - with several million more to follow in weeks - but the original expectation that there could be 10 million doses by the end of the year is already looking ambitious.
Getting the jabs into the country remains a challenge. It is being made in Belgium. Could Brexit be an issue? The government is confident it has secure routes to ensure supply does not get disrupted.
Nonetheless, major hopes are still being pinned to authorisation being given to the Oxford University vaccine so that rollout can happen as quickly as possible in 2021.
The order in which people will get the jab is decided by the Joint Committee on Vaccinations and Immunisations.
Mass immunisation of everyone over 50, as well as younger people with pre-existing health conditions, can happen as more stocks become available in 2021.
The vaccine is given as two injections, 21 days apart, with the second dose being a booster. Immunity begins to kick in after the first dose but reaches its full effect seven days after the second dose.
Most of the side effects are very mild, similar to the side effects after any other vaccine and usually last for a day or so, said Prof Sir Munir Pirmohamed, the chairman of the Commission on Human Medicine expert working group.
The vaccine was 95% effective for all groups in the trials, including elderly people, he said.
The head of the MHRA, Dr June Raine, said despite the speed of approval, no corners have been cut.
Batches of the vaccine will be tested in labs "so that every single vaccine that goes out meets the same high standards of safety", she said.
media captionDr June Raine from the MHRA: "The safety of the public will always come first"
Giving the analogy of climbing a mountain, she said: "If you're climbing a mountain, you prepare and prepare. We started that in June. By the time the interim results became available on 10 November we were at base camp.
"And then when we got the final analysis we were ready for that last sprint that takes us to today."
The Pfizer/BioNTech was the first vaccine to publish positive early results from final stages of testing.
It is a new type called an mRNA vaccine that uses a tiny fragment of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
An mRNA vaccine has never been approved for use in humans before, although people have received them in clinical trials.
Because the vaccine must be stored at around -70C, it will be transported in special boxes of up to 5,000 doses, packed in dry ice.
Once delivered, it can be kept for up to five days in a fridge. And once out of the fridge it needs to be used within six hours.
There are some other promising vaccines that could also be approved soon.
One from Moderna uses the same mRNA approach as the Pfizer vaccine and offers similar protection. The UK has pre-ordered seven million doses that could be ready by the spring.
The UK has ordered 100 million doses of a different type of Covid vaccine from Oxford University and AstraZeneca. That vaccine uses a harmless virus, altered to look a lot more like the virus that causes Covid-19.
Russia has been using another vaccine, called Sputnik, and the Chinese military has approved another one made by CanSino Biologics. Both work in a similar way to the Oxford vaccine
https://www.bbc.co.uk/news/health-55145696
safe for use in UK from next week
The UK has become the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine, paving the way for mass vaccination
- NOT IN MY ARM
The first doses are already on their way to the UK, with 800,000 due in the coming days, Pfizer said.
Health Secretary Matt Hancock said the NHS will contact people about jabs.
Elderly people in care homes and care home staff have been placed top of the priority list, followed by over-80s and health and care staff.
But because hospitals already have the facilities to store the vaccine at -70C, as required, the very first vaccinations are likely to take place there - for care home staff, NHS staff and patients - so none of the vaccine is wasted.
The Pfizer/BioNTech jab is the fastest vaccine to go from concept to reality, taking only 10 months to follow the same steps that normally span 10 years.
The UK has already ordered 40 million doses of the jab - enough to vaccinate 20 million people.
The doses will be rolled out as quickly as they can be made by Pfizer in Belgium, Mr Hancock said, with the first load next week and then "several millions" throughout December.
Scottish First Minister Nicola Sturgeon said the first people in Scotland will be immunised on Tuesday.
The bulk of the rollout will be next year, Mr Hancock said. "2020 has been just awful and 2021 is going to be better," he said.
"I'm confident now, with the news today, that from spring, from Easter onwards, things are going to be better. And we're going to have a summer next year that everybody can enjoy."
Prime Minister Boris Johnson added: "It's the protection of vaccines that will ultimately allow us to reclaim our lives and get the economy moving again."
media captionHeath Secretary Matt Hancock says he is "thrilled" that the vaccine has been approved
The free vaccine will not be compulsory and there will be three ways of vaccinating people across the UK:
- Hospitals
in the community, with GPs and pharmacists
Vaccination centres a bit like the Nightingales project and including some of the Nightingales
It is thought the vaccination network could start delivering more than one million doses a week once enough doses are available.
NHS chief executive Sir Simon Stevens said the health service was preparing for "the largest-scale vaccination campaign in our country's history".
But experts said people still need to remain vigilant and follow rules to stop the virus spreading - including with social distancing, face masks and self-isolation.
"We can't lower our guard yet," said the government's chief medical adviser Prof Chris Whitty.
This is the news we have all been waiting for
Analysis box by Nick Triggle, health correspondent
This is the news we have all been waiting for.
The NHS has already been gearing up for this moment for some time. The venues are in place and there is provision for extra staffing, with even lifeguards and airline staff to be brought in to help with the effort.
But the biggest hurdle will be supply.
The UK has been promised 40 million doses by the spring - enough to give the required two jabs to health and care workers and everyone over 65. But in the first few weeks of winter, our ability to vaccinate could easily outstrip supply.
Ministers say they will have 800,000 doses in the country within days - with several million more to follow in weeks - but the original expectation that there could be 10 million doses by the end of the year is already looking ambitious.
Getting the jabs into the country remains a challenge. It is being made in Belgium. Could Brexit be an issue? The government is confident it has secure routes to ensure supply does not get disrupted.
Nonetheless, major hopes are still being pinned to authorisation being given to the Oxford University vaccine so that rollout can happen as quickly as possible in 2021.
The order in which people will get the jab is decided by the Joint Committee on Vaccinations and Immunisations.
Mass immunisation of everyone over 50, as well as younger people with pre-existing health conditions, can happen as more stocks become available in 2021.
The vaccine is given as two injections, 21 days apart, with the second dose being a booster. Immunity begins to kick in after the first dose but reaches its full effect seven days after the second dose.
Most of the side effects are very mild, similar to the side effects after any other vaccine and usually last for a day or so, said Prof Sir Munir Pirmohamed, the chairman of the Commission on Human Medicine expert working group.
The vaccine was 95% effective for all groups in the trials, including elderly people, he said.
The head of the MHRA, Dr June Raine, said despite the speed of approval, no corners have been cut.
Batches of the vaccine will be tested in labs "so that every single vaccine that goes out meets the same high standards of safety", she said.
media captionDr June Raine from the MHRA: "The safety of the public will always come first"
Giving the analogy of climbing a mountain, she said: "If you're climbing a mountain, you prepare and prepare. We started that in June. By the time the interim results became available on 10 November we were at base camp.
"And then when we got the final analysis we were ready for that last sprint that takes us to today."
The Pfizer/BioNTech was the first vaccine to publish positive early results from final stages of testing.
It is a new type called an mRNA vaccine that uses a tiny fragment of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
An mRNA vaccine has never been approved for use in humans before, although people have received them in clinical trials.
Because the vaccine must be stored at around -70C, it will be transported in special boxes of up to 5,000 doses, packed in dry ice.
Once delivered, it can be kept for up to five days in a fridge. And once out of the fridge it needs to be used within six hours.
There are some other promising vaccines that could also be approved soon.
One from Moderna uses the same mRNA approach as the Pfizer vaccine and offers similar protection. The UK has pre-ordered seven million doses that could be ready by the spring.
The UK has ordered 100 million doses of a different type of Covid vaccine from Oxford University and AstraZeneca. That vaccine uses a harmless virus, altered to look a lot more like the virus that causes Covid-19.
Russia has been using another vaccine, called Sputnik, and the Chinese military has approved another one made by CanSino Biologics. Both work in a similar way to the Oxford vaccine
https://www.bbc.co.uk/news/health-55145696
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Oxford Vaccine
How did they make it so quickly?
Ten years' vaccine work achieved in about 10 months. Yet no corners cut in designing, testing and manufacturing !?!
They are two statements that sound like a contradiction, and have led some to ask how we can be sure the Oxford vaccine - which has published its first results showing it is highly effective at stopping Covid-19 - is safe when it has been made so fast.
So, this is the real story of how the Oxford vaccine happened so quickly.
It is one that relies on good fortune as well as scientific brilliance; has origins in both a deadly Ebola outbreak and a chimpanzee's runny nose; and sees the researchers go from having no money in the bank to chartering private planes.
The work started years ago
We were planning how can we go really quickly to have a vaccine in someone in the shortest possible time
The biggest misconception is the work on the vaccine started when the pandemic began.
The world's biggest Ebola outbreak in 2014-2016 was a catastrophe. The response was too slow and 11,000 people died.
"The world should have done better," Prof Sarah Gilbert, the architect of the Oxford vaccine, told me.
In the recriminations that followed, a plan emerged for how to tackle the next big one. At the end of a list of known threats was "Disease X" - the sinister name of a new, unknown infection that would take the world by surprise.
The Jenner Institute at the University of Oxford - named after the scientist that performed the first vaccination in 1796, and now home to some of the world's leading experts - designed a strategy for defeating an unknown enemy.
"We were planning how can we go really quickly to have a vaccine in someone in the shortest possible time," Prof Gilbert said.
"We hadn't got the plan finished, but we did do pretty well."
The critical piece of technology
The central piece of their plan was a revolutionary style of vaccine known as "plug and play". It has two highly desirable traits for facing the unknown - it is both fast and flexible.
Conventional vaccines - including the whole of the childhood immunisation programme - use a killed or weakened form of the original infection, or inject fragments of it into the body. But these are slow to develop.
Instead the Oxford researchers constructed ChAdOx1 - or Chimpanzee Adenovirus Oxford One.
Scientists took a common cold virus that infected chimpanzees and engineered it to become the building block of a vaccine against almost anything.
Before Covid, 330 people had been given ChAdOx1 based-vaccines for diseases ranging from flu to Zika virus, and prostate cancer to the tropical disease chikungunya.
The virus from chimps is genetically modified so it cannot cause an infection in people. It can then be modified again to contain the genetic blueprints for whatever you want to train the immune system to attack. This target is known is an antigen.
ChAdOx1 is in essence a sophisticated, microscopic postman. All the scientists have to do is change the package.
"We drop it in and off we go," said Prof Gilbert.
January 1
We'd been planning for disease X, we'd been waiting for disease X, and I thought this could be it.
While much of the world was having a lie-in after New Year's Eve, Prof Gilbert noticed concerning reports of "viral pneumonia" in Wuhan, China. Within two weeks scientists had identified the virus responsible and began to suspect it was able to spread between people.
"We'd been planning for disease X, we'd been waiting for disease X, and I thought this could be it," Prof Gilbert said.
At this point, the team did not know how important their work would become. It started out as a test of how fast they could go and as a demonstration of the ChAdOx1 technology.
Prof Gilbert said: "I thought it might only have been a project, we'd make the vaccine and the virus would fizzle out. But it didn't."
A lucky break
It sounds strange to say it, almost perverse, but it was lucky that the pandemic was caused by a coronavirus.
This family of viruses had tried to jump from animals to people twice before in the past 20 years - Sars coronavirus in 2002 and Mers coronavirus in 2012.
It meant scientists knew the virus's biology, how it behaved and its Achilles heel - the "spike protein".
"We had a huge head start," Prof Andrew Pollard from the Oxford team said.
The spike protein is the key the virus uses to unlock the doorway into our body's cells. If a vaccine could train the immune system to attack the spike, then the team knew they were odds-on to succeed.
And they had already developed a ChAdOx1 vaccine for Mers, which could train the immune system to spot the spike. The Oxford team were not starting from scratch.
"If this had been a completely unknown virus, then we'd have been in a very different position," Prof Pollard added.
It was also lucky that coronaviruses cause short-term infections. It means the body is capable of beating the virus and a vaccine just needs to tap into that natural process.
If it had been a long-term or chronic infection that the body cannot beat - like HIV - then it's unlikely a vaccine could work.
On 11 January, Chinese scientists published and shared with the world the full genetic code of the coronavirus.
The team now had everything they needed to make a Covid-19 vaccine.
All they had to do was slip the genetic instructions for the spike protein into ChAdOx1 and they were good to go.
Money, money, money
Making a vaccine is very expensive.
"The first bit was quite painful. There was a period when we didn't have any money in the bank," said Prof Pollard.
They had some funding from the university, but they had a crucial advantage over other groups around the world.
On the site of Churchill hospital in Oxford is the group's own vaccine manufacturing plant.
"We could say stop everything else and make this vaccine," said Prof Pollard.
It was enough to get going, but not to make the thousands of doses needed for larger trials.
"Getting money was my main activity until April, just trying to persuade people to fund it now," said Prof Gilbert.
But as the pandemic tightened its grip on the world and country after country descended into lockdown, the money started to flow. Production of the vaccine moved to a facility in Italy and the money helped solve problems that would have otherwise held up the trials, including the logistical nightmare of lockdown Europe.
"At one point we had to charter a plane, the vaccine was in Italy and we had clinics here the next morning," said Prof Gilbert.
Unglamorous, but vital checks
Quality control is never the sexiest part of a project, but researchers cannot start giving an experimental vaccine to people until they are sure it has been made to a high enough standard.
At every stage of the manufacturing process, they needed to ensure the vaccine was not being contaminated with viruses or bacteria. In the past this had been a lengthy process.
"If we had not been thinking about how to shorten the time, we might have had a vaccine in March but not started trials until June."
Instead, once animal trials had shown the vaccine was safe, the researchers were able to begin human trials of the vaccine on 23 April.
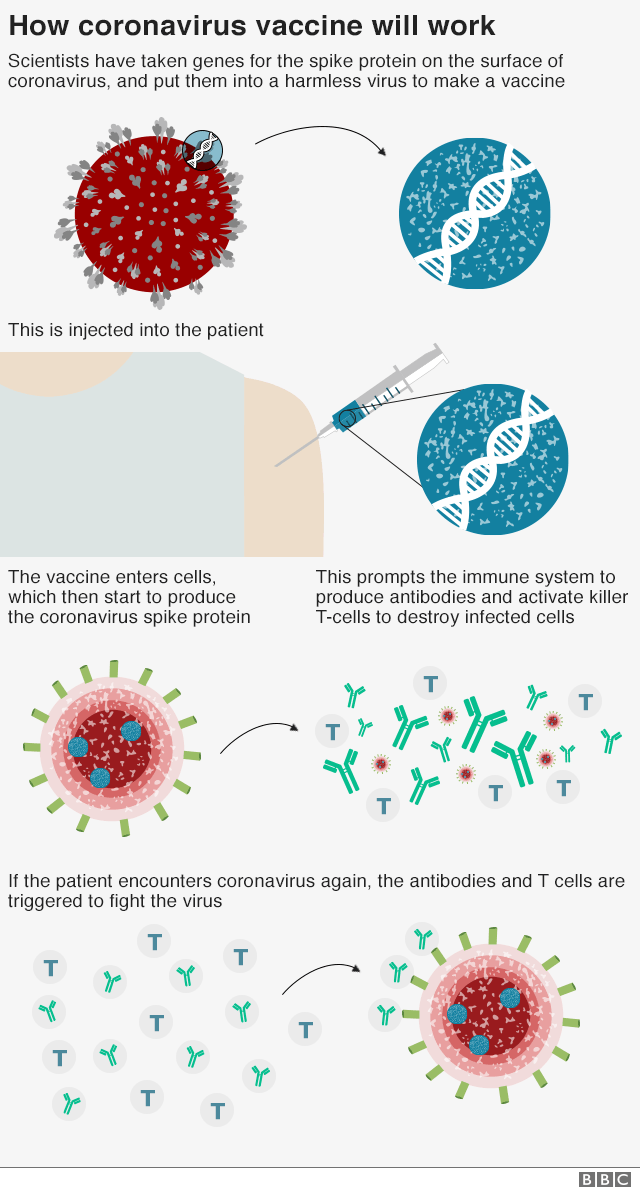
Back-to-back trials
Since then the Oxford vaccine has been through every stage of trials that would normally take place for a vaccine.
There is a pattern to clinical trials:
What hasn't happened is years of hanging around in between each phase.
The process is long, not because it needs to be and not because it's safe, but because of the real world.
Dr Mark Toshner, who has been involved in the trials at sites in Cambridge, said the idea that it took 10 years to trial a vaccine was misleading.
He told the BBC: "Most of the time, it's a lot of nothing."
He describes it as a process of writing grant applications, having them rejected, writing them again, getting approval to do the trial, negotiating with manufacturers, and trying to recruit enough people to take part. It can take years to get from one phase to the next.
"The process is long, not because it needs to be and not because it's safe, but because of the real world," Dr Toshner said.
Safety has not been sacrificed. Instead the unparalleled scientific push to make the trials happen, the droves of people willing to take part, and of course the money blew many of the usual hold-ups aside.
That does not mean problems will not appear in the future - medical research cannot make those guarantees. Usually, side-effects of vaccines appear either at the time they are given or a few months afterwards. It is possible that rarer problems could emerge when millions of people are immunised, but this is true of every vaccine that has ever been developed.
The next stage will be fast too
Plans for giving regulatory approval and manufacturing the vaccine have also been dramatically speeded up.
The UK already has four million doses of the vaccine ready to deploy. The Oxford team partnered with pharmaceutical giant AstraZeneca, and the manufacture of the vaccine started long before the results came in. At the time it was a gamble, but it has paid off big time.
Regulators, who would normally wait until after the trials were concluded, have also been involved early.
The Medicines and Healthcare products Regulatory Agency in the UK has been conducting "rolling reviews" of the safety, manufacturing standards and effectiveness of the Oxford vaccine. It means a decision on whether the vaccine can be used will come early.
The Oxford vaccine has - like those of Pfizer and Moderna - arrived in record time to a world in desperate need.
https://www.bbc.co.uk/news/health-55041371
How did they make it so quickly?
Ten years' vaccine work achieved in about 10 months. Yet no corners cut in designing, testing and manufacturing !?!
They are two statements that sound like a contradiction, and have led some to ask how we can be sure the Oxford vaccine - which has published its first results showing it is highly effective at stopping Covid-19 - is safe when it has been made so fast.
So, this is the real story of how the Oxford vaccine happened so quickly.
It is one that relies on good fortune as well as scientific brilliance; has origins in both a deadly Ebola outbreak and a chimpanzee's runny nose; and sees the researchers go from having no money in the bank to chartering private planes.
The work started years ago
We were planning how can we go really quickly to have a vaccine in someone in the shortest possible time
The biggest misconception is the work on the vaccine started when the pandemic began.
The world's biggest Ebola outbreak in 2014-2016 was a catastrophe. The response was too slow and 11,000 people died.
"The world should have done better," Prof Sarah Gilbert, the architect of the Oxford vaccine, told me.
In the recriminations that followed, a plan emerged for how to tackle the next big one. At the end of a list of known threats was "Disease X" - the sinister name of a new, unknown infection that would take the world by surprise.
The Jenner Institute at the University of Oxford - named after the scientist that performed the first vaccination in 1796, and now home to some of the world's leading experts - designed a strategy for defeating an unknown enemy.
"We were planning how can we go really quickly to have a vaccine in someone in the shortest possible time," Prof Gilbert said.
"We hadn't got the plan finished, but we did do pretty well."
The critical piece of technology
The central piece of their plan was a revolutionary style of vaccine known as "plug and play". It has two highly desirable traits for facing the unknown - it is both fast and flexible.
Conventional vaccines - including the whole of the childhood immunisation programme - use a killed or weakened form of the original infection, or inject fragments of it into the body. But these are slow to develop.
Instead the Oxford researchers constructed ChAdOx1 - or Chimpanzee Adenovirus Oxford One.
Scientists took a common cold virus that infected chimpanzees and engineered it to become the building block of a vaccine against almost anything.
Before Covid, 330 people had been given ChAdOx1 based-vaccines for diseases ranging from flu to Zika virus, and prostate cancer to the tropical disease chikungunya.
The virus from chimps is genetically modified so it cannot cause an infection in people. It can then be modified again to contain the genetic blueprints for whatever you want to train the immune system to attack. This target is known is an antigen.
ChAdOx1 is in essence a sophisticated, microscopic postman. All the scientists have to do is change the package.
"We drop it in and off we go," said Prof Gilbert.
January 1
We'd been planning for disease X, we'd been waiting for disease X, and I thought this could be it.
While much of the world was having a lie-in after New Year's Eve, Prof Gilbert noticed concerning reports of "viral pneumonia" in Wuhan, China. Within two weeks scientists had identified the virus responsible and began to suspect it was able to spread between people.
"We'd been planning for disease X, we'd been waiting for disease X, and I thought this could be it," Prof Gilbert said.
At this point, the team did not know how important their work would become. It started out as a test of how fast they could go and as a demonstration of the ChAdOx1 technology.
Prof Gilbert said: "I thought it might only have been a project, we'd make the vaccine and the virus would fizzle out. But it didn't."
A lucky break
It sounds strange to say it, almost perverse, but it was lucky that the pandemic was caused by a coronavirus.
This family of viruses had tried to jump from animals to people twice before in the past 20 years - Sars coronavirus in 2002 and Mers coronavirus in 2012.
It meant scientists knew the virus's biology, how it behaved and its Achilles heel - the "spike protein".
"We had a huge head start," Prof Andrew Pollard from the Oxford team said.
The spike protein is the key the virus uses to unlock the doorway into our body's cells. If a vaccine could train the immune system to attack the spike, then the team knew they were odds-on to succeed.
And they had already developed a ChAdOx1 vaccine for Mers, which could train the immune system to spot the spike. The Oxford team were not starting from scratch.
"If this had been a completely unknown virus, then we'd have been in a very different position," Prof Pollard added.
It was also lucky that coronaviruses cause short-term infections. It means the body is capable of beating the virus and a vaccine just needs to tap into that natural process.
If it had been a long-term or chronic infection that the body cannot beat - like HIV - then it's unlikely a vaccine could work.
On 11 January, Chinese scientists published and shared with the world the full genetic code of the coronavirus.
The team now had everything they needed to make a Covid-19 vaccine.
All they had to do was slip the genetic instructions for the spike protein into ChAdOx1 and they were good to go.
Money, money, money
Making a vaccine is very expensive.
"The first bit was quite painful. There was a period when we didn't have any money in the bank," said Prof Pollard.
They had some funding from the university, but they had a crucial advantage over other groups around the world.
On the site of Churchill hospital in Oxford is the group's own vaccine manufacturing plant.
"We could say stop everything else and make this vaccine," said Prof Pollard.
It was enough to get going, but not to make the thousands of doses needed for larger trials.
"Getting money was my main activity until April, just trying to persuade people to fund it now," said Prof Gilbert.
But as the pandemic tightened its grip on the world and country after country descended into lockdown, the money started to flow. Production of the vaccine moved to a facility in Italy and the money helped solve problems that would have otherwise held up the trials, including the logistical nightmare of lockdown Europe.
"At one point we had to charter a plane, the vaccine was in Italy and we had clinics here the next morning," said Prof Gilbert.
Unglamorous, but vital checks
Quality control is never the sexiest part of a project, but researchers cannot start giving an experimental vaccine to people until they are sure it has been made to a high enough standard.
At every stage of the manufacturing process, they needed to ensure the vaccine was not being contaminated with viruses or bacteria. In the past this had been a lengthy process.
"If we had not been thinking about how to shorten the time, we might have had a vaccine in March but not started trials until June."
Instead, once animal trials had shown the vaccine was safe, the researchers were able to begin human trials of the vaccine on 23 April.
- There should never be trials on innocent animals, use the murderers, drug dealers, rapists, child molesters and ISIS supporters in world prisons

Back-to-back trials
Since then the Oxford vaccine has been through every stage of trials that would normally take place for a vaccine.
There is a pattern to clinical trials:
- Phase one - the vaccine is tested in a small number of people to check it is safe
Phase two - safety tests in more people, and to look for signs the vaccine is producing the required response
Phase three - the big trial, involving thousands of people, to prove it actually protects people
What hasn't happened is years of hanging around in between each phase.
The process is long, not because it needs to be and not because it's safe, but because of the real world.
Dr Mark Toshner, who has been involved in the trials at sites in Cambridge, said the idea that it took 10 years to trial a vaccine was misleading.
He told the BBC: "Most of the time, it's a lot of nothing."
He describes it as a process of writing grant applications, having them rejected, writing them again, getting approval to do the trial, negotiating with manufacturers, and trying to recruit enough people to take part. It can take years to get from one phase to the next.
"The process is long, not because it needs to be and not because it's safe, but because of the real world," Dr Toshner said.
Safety has not been sacrificed. Instead the unparalleled scientific push to make the trials happen, the droves of people willing to take part, and of course the money blew many of the usual hold-ups aside.
That does not mean problems will not appear in the future - medical research cannot make those guarantees. Usually, side-effects of vaccines appear either at the time they are given or a few months afterwards. It is possible that rarer problems could emerge when millions of people are immunised, but this is true of every vaccine that has ever been developed.
The next stage will be fast too
Plans for giving regulatory approval and manufacturing the vaccine have also been dramatically speeded up.
The UK already has four million doses of the vaccine ready to deploy. The Oxford team partnered with pharmaceutical giant AstraZeneca, and the manufacture of the vaccine started long before the results came in. At the time it was a gamble, but it has paid off big time.
Regulators, who would normally wait until after the trials were concluded, have also been involved early.
The Medicines and Healthcare products Regulatory Agency in the UK has been conducting "rolling reviews" of the safety, manufacturing standards and effectiveness of the Oxford vaccine. It means a decision on whether the vaccine can be used will come early.
The Oxford vaccine has - like those of Pfizer and Moderna - arrived in record time to a world in desperate need.
https://www.bbc.co.uk/news/health-55041371
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
When can I get the Pfizer vaccine?
The Pfizer/BioNTech coronavirus vaccine has been approved for widespread use in the UK
What is the Pfizer vaccine and how does it work?
The vaccine trains the immune system to fight coronavirus.
It is a new type of jab called an RNA vaccine and uses a tiny fragment of the virus's genetic code. This starts making part of the virus inside the body, which the immune system recognises as foreign and starts to attack.
The genetic material is encased in a tiny protective bubble of fat to get it into cells.
The exact ingredients of the vaccine have not been made public, but other vaccines can contain other ingredients, like aluminium, to make them stable or more effective.
The vaccine is given in two doses - three weeks apart - and offers up to 95% protection against Covid-19.
How will the new Pfizer vaccine work?
Has this type of vaccine ever been used before?
This is the first RNA vaccine to be approved for use in humans.
The concept has been researched before and people have been given them in clinical trials for other diseases.
The vaccine will be considered by regulatory agencies around the world, who will decide whether the jab can be approved for use.
Who will get it first and how soon can I have it?
In the UK, older care home residents and their carers are top of the priority list in the first phase of the vaccination programme. They are followed by frontline health and social care workers and the over-80s.
Out of the nine priority groups, those aged 50-54 are at the bottom of the current list.
The first jabs may take place within days now approval has been given.
There will be a second phase, which will offer the vaccine to the other groups in the population.
The vaccine will be delivered through care homes, GPs and pharmacists as well as "go-to" vaccination centres set up in venues like sports halls.
However, there are logistical challenges to overcome:
It must be kept at -70C during transportation
The jab must be thawed before it is given to a patient
It can be stored in a normal fridge for a few days before being used
Graphic outlining how the Pfizer vaccine will be prioritised among different groups
Will it offer lasting protection?
It is impossible to know and we will find out only by waiting.
If immunity does not last then it may be necessary to have annual vaccines, as we do for flu.
The vaccine appears to protect 94% of adults over 65 years old and data from its phase three trial suggests it works equally well in people of all ages and ethnicities.
Some people - such as those with a weak immune system - will not be able to have the vaccine.
Could the vaccine have long-term side effects?
Nothing in medicine is 100% safe - even something we take without thinking, like paracetamol, poses risks.
The data so far is reassuring - trials on 43,500 people discovered no safety concerns, although mild side effects, such as headaches and muscle aches, have been reported.
If there were highly dangerous and common consequences of this vaccination, they should have become apparent.
However, rarer side effects may emerge as millions of people are immunised.
Will it mean we don't need lockdown?
Hopefully yes, but not for some time.
If enough people are immune then the virus would stop spreading and we would not need other measures.
However, the manufacture and distribution of a vaccine will take some time.
So testing, lockdowns, social distancing, and mask wearing are going to be a feature of our lives for a while yet.
Why can it only be made by Pfizer?
The vaccine has been designed and developed by Pfizer and BioNtech, and they own the intellectual property.
They already have the manufacturing capacity to produce 1.3 billion doses by the end of next year, but could partner with others to increase capacity even further.
What do we still need to know about the vaccine?
The announcement gave us the headline, but there is a still lack of fine detail.
We do not know if the vaccine stops you catching and spreading the virus or just stops you from getting ill. We also don't know how protective the vaccine is in different age groups.
These will be crucial for understanding how it will be used.
What does this mean for other vaccines?
It is good news - it shows that a coronavirus vaccine is possible,.
About a dozen vaccines are in the final development stages and those produced by Oxford University/AstraZeneca and Moderna have also proved successful in trials. The UK has ordered supplies of both.
https://www.bbc.co.uk/news/explainers-54880084
The Pfizer/BioNTech coronavirus vaccine has been approved for widespread use in the UK
What is the Pfizer vaccine and how does it work?
The vaccine trains the immune system to fight coronavirus.
It is a new type of jab called an RNA vaccine and uses a tiny fragment of the virus's genetic code. This starts making part of the virus inside the body, which the immune system recognises as foreign and starts to attack.
The genetic material is encased in a tiny protective bubble of fat to get it into cells.
The exact ingredients of the vaccine have not been made public, but other vaccines can contain other ingredients, like aluminium, to make them stable or more effective.
The vaccine is given in two doses - three weeks apart - and offers up to 95% protection against Covid-19.
How will the new Pfizer vaccine work?
Has this type of vaccine ever been used before?
This is the first RNA vaccine to be approved for use in humans.
The concept has been researched before and people have been given them in clinical trials for other diseases.
The vaccine will be considered by regulatory agencies around the world, who will decide whether the jab can be approved for use.
Who will get it first and how soon can I have it?
In the UK, older care home residents and their carers are top of the priority list in the first phase of the vaccination programme. They are followed by frontline health and social care workers and the over-80s.
Out of the nine priority groups, those aged 50-54 are at the bottom of the current list.
The first jabs may take place within days now approval has been given.
There will be a second phase, which will offer the vaccine to the other groups in the population.
The vaccine will be delivered through care homes, GPs and pharmacists as well as "go-to" vaccination centres set up in venues like sports halls.
However, there are logistical challenges to overcome:
It must be kept at -70C during transportation
The jab must be thawed before it is given to a patient
It can be stored in a normal fridge for a few days before being used
Graphic outlining how the Pfizer vaccine will be prioritised among different groups
Will it offer lasting protection?
It is impossible to know and we will find out only by waiting.
If immunity does not last then it may be necessary to have annual vaccines, as we do for flu.
The vaccine appears to protect 94% of adults over 65 years old and data from its phase three trial suggests it works equally well in people of all ages and ethnicities.
Some people - such as those with a weak immune system - will not be able to have the vaccine.
Could the vaccine have long-term side effects?
Nothing in medicine is 100% safe - even something we take without thinking, like paracetamol, poses risks.
The data so far is reassuring - trials on 43,500 people discovered no safety concerns, although mild side effects, such as headaches and muscle aches, have been reported.
If there were highly dangerous and common consequences of this vaccination, they should have become apparent.
However, rarer side effects may emerge as millions of people are immunised.
Will it mean we don't need lockdown?
Hopefully yes, but not for some time.
If enough people are immune then the virus would stop spreading and we would not need other measures.
However, the manufacture and distribution of a vaccine will take some time.
So testing, lockdowns, social distancing, and mask wearing are going to be a feature of our lives for a while yet.
Why can it only be made by Pfizer?
The vaccine has been designed and developed by Pfizer and BioNtech, and they own the intellectual property.
They already have the manufacturing capacity to produce 1.3 billion doses by the end of next year, but could partner with others to increase capacity even further.
What do we still need to know about the vaccine?
The announcement gave us the headline, but there is a still lack of fine detail.
We do not know if the vaccine stops you catching and spreading the virus or just stops you from getting ill. We also don't know how protective the vaccine is in different age groups.
These will be crucial for understanding how it will be used.
What does this mean for other vaccines?
It is good news - it shows that a coronavirus vaccine is possible,.
About a dozen vaccines are in the final development stages and those produced by Oxford University/AstraZeneca and Moderna have also proved successful in trials. The UK has ordered supplies of both.
https://www.bbc.co.uk/news/explainers-54880084
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Allergy warning over new jab
People with a history of significant allergic reactions should not have the Pfizer/BioNTech Covid jab
It came after two NHS workers had allergic reactions on Tuesday.
The advice applies to those who have had reactions to medicines, food or vaccines, the Medicines and Healthcare products Regulatory Agency said.
The two people had a reaction shortly after having the new jab, had treatment and are both fine now.
They are understood to have had an anaphylactoid reaction, which tends to involve a skin rash, breathlessness and sometimes a drop in blood pressure. This is not the same as anaphylaxis which can be fatal.
Both NHS workers have a history of serious allergies and carry adrenaline pens around with them.
Professor Stephen Powis, medical director for the NHS in England, said both individuals were recovering well.
He said this was "common with new vaccines", describing it as a precautionary measure.
Dr June Raine, head of the MHRA, said it was only right to take this step now that "we've had this experience".
Reactions like this are uncommon, but do happen with other vaccines, including the annual flu jab.
Several thousand people were vaccinated on Tuesday in hospital clinics on the first day of the UK rollout of the new Covid jab.
Prof Peter Openshaw, an expert in immunology at Imperial College London, said: "The fact that we know so soon about these two allergic reactions and that the regulator has acted on this to issue precautionary advice shows that this monitoring system is working well."
This is a story to assess with your head and not your gut
No effective medicine is without side effects so you have to balance the risk and the benefit.
Remember, one in a thousand people in the UK have died after being infected with coronavirus this year and that figure is rising daily.
Two people, out of thousands vaccinated yesterday, had an allergic reaction which they recovered from.
Such reactions can happen with any vaccine and are treated with drugs such as steroids or adrenaline.
The trials reported one possible allergic reaction per thousand people immunised that may have been related to the jab.
The MHRA has given targeted advice to those most at risk, but for the overwhelming majority of people, this changes nothing.
GPs 'ready to go' for community rollout
The development came after the NHS has announced the vaccination programme will be expanded out to GP surgeries from next week.
Doses are expected to be delivered to around 200 GP surgeries initially to allow them to start on Tuesday. The over-80s will be invited first.
Once the first 200 GP practices have received their doses the programme will be expanded out to more than 1,000 surgeries - with each local area having a designated site.
It means most patients will be invited to a GP centre that is not their usual one.
Similar arrangements are being made in the rest of the UK.
How the Pfizer vaccine requires two doses
Dr Richard Vautrey, GP leader from the British Medical Association, said GPs were "ready to go".
"We have a wealth of experience in delivering vaccines - and will be able to do millions of people a week. It is really dependent on supply and how quickly we can get our hands on it."
Second Covid vaccine 'effective in elderly'
Meanwhile, one of the key people behind another Covid vaccine developed by Oxford University and AstraZeneca has played down fears the jab may not be effective in older people.
Data published by the Lancet on Tuesday suggested there was a lack of certainty over the effectiveness of the vaccine in the over-55s.
Prof Sarah Gilbert said older people were only recruited into the trials later in the process, and the next trial results provided were likely to include information about how well the jab worked in people over the age of 55.
But she said there "was no difference" in the immune response seen in younger adults and people over 70 in earlier trials.
This meant the regulator could support licensing of the vaccine to the elderly, she said.
The vaccine is crucial to fast rollout as it is much easier to store and distribute, because it does not need to be kept at ultra-cold temperatures. There are more than 5 million doses of the vaccine currently in the country.
The latest daily figures for the UK, published on Wednesday, showed a further 533 people have now died within 28 days of a positive Covid-19 test. A further 16,578 people have tested positive for Covid-19, taking the UK's total cases to 1,766,819.
https://www.bbc.co.uk/news/health-55244122
People with a history of significant allergic reactions should not have the Pfizer/BioNTech Covid jab
It came after two NHS workers had allergic reactions on Tuesday.
The advice applies to those who have had reactions to medicines, food or vaccines, the Medicines and Healthcare products Regulatory Agency said.
The two people had a reaction shortly after having the new jab, had treatment and are both fine now.
They are understood to have had an anaphylactoid reaction, which tends to involve a skin rash, breathlessness and sometimes a drop in blood pressure. This is not the same as anaphylaxis which can be fatal.
Both NHS workers have a history of serious allergies and carry adrenaline pens around with them.
Professor Stephen Powis, medical director for the NHS in England, said both individuals were recovering well.
He said this was "common with new vaccines", describing it as a precautionary measure.
Dr June Raine, head of the MHRA, said it was only right to take this step now that "we've had this experience".
Reactions like this are uncommon, but do happen with other vaccines, including the annual flu jab.
Several thousand people were vaccinated on Tuesday in hospital clinics on the first day of the UK rollout of the new Covid jab.
Prof Peter Openshaw, an expert in immunology at Imperial College London, said: "The fact that we know so soon about these two allergic reactions and that the regulator has acted on this to issue precautionary advice shows that this monitoring system is working well."
This is a story to assess with your head and not your gut
No effective medicine is without side effects so you have to balance the risk and the benefit.
Remember, one in a thousand people in the UK have died after being infected with coronavirus this year and that figure is rising daily.
Two people, out of thousands vaccinated yesterday, had an allergic reaction which they recovered from.
Such reactions can happen with any vaccine and are treated with drugs such as steroids or adrenaline.
The trials reported one possible allergic reaction per thousand people immunised that may have been related to the jab.
The MHRA has given targeted advice to those most at risk, but for the overwhelming majority of people, this changes nothing.
GPs 'ready to go' for community rollout
The development came after the NHS has announced the vaccination programme will be expanded out to GP surgeries from next week.
Doses are expected to be delivered to around 200 GP surgeries initially to allow them to start on Tuesday. The over-80s will be invited first.
Once the first 200 GP practices have received their doses the programme will be expanded out to more than 1,000 surgeries - with each local area having a designated site.
It means most patients will be invited to a GP centre that is not their usual one.
Similar arrangements are being made in the rest of the UK.
How the Pfizer vaccine requires two doses
Dr Richard Vautrey, GP leader from the British Medical Association, said GPs were "ready to go".
"We have a wealth of experience in delivering vaccines - and will be able to do millions of people a week. It is really dependent on supply and how quickly we can get our hands on it."
Second Covid vaccine 'effective in elderly'
Meanwhile, one of the key people behind another Covid vaccine developed by Oxford University and AstraZeneca has played down fears the jab may not be effective in older people.
Data published by the Lancet on Tuesday suggested there was a lack of certainty over the effectiveness of the vaccine in the over-55s.
Prof Sarah Gilbert said older people were only recruited into the trials later in the process, and the next trial results provided were likely to include information about how well the jab worked in people over the age of 55.
But she said there "was no difference" in the immune response seen in younger adults and people over 70 in earlier trials.
This meant the regulator could support licensing of the vaccine to the elderly, she said.
The vaccine is crucial to fast rollout as it is much easier to store and distribute, because it does not need to be kept at ultra-cold temperatures. There are more than 5 million doses of the vaccine currently in the country.
The latest daily figures for the UK, published on Wednesday, showed a further 533 people have now died within 28 days of a positive Covid-19 test. A further 16,578 people have tested positive for Covid-19, taking the UK's total cases to 1,766,819.
https://www.bbc.co.uk/news/health-55244122
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Efforts to Trace Origin of Covid
With cases soaring in the United States and elsewhere, the Covid-19 pandemic is nowhere near its end
With three vaccines reporting trial data and two apparently nearing approval by the US Food and Drug Administration, it may be reaching a pivot point. In what feels like a moment of drawing breath and taking stock, international researchers are turning their attention from the present back to the start of the pandemic, aiming to untangle its origin and asking what lessons can be learned to keep this from happening again.
Two efforts are happening in parallel. On November 5, the World Health Organization quietly published the rules of engagement for a long-planned and months-delayed mission that creates a multinational team of researchers who will pursue how the virus leaped species. Meanwhile, last week, a commission created by The Lancet and headed by the economist and policy expert Jeffrey Sachs announced the formation of its own international effort, a task force of 12 experts from nine countries who will undertake similar tasks.
Both groups will face the same complex problems. It has been approximately a year since the first cases of a pneumonia of unknown origin appeared in Wuhan, China, and about 11 months since the pneumonia’s cause was identified as a novel coronavirus, probably originating in bats. The experts will have to retrace a chain of transmission—one or multiple leaps of the virus from the animal world into humans—using interviews, stored biological samples, lab assays, environmental surveys, genomic data, and the thousands of papers published since the pandemic began, all while following a trail that may have gone cold.
The point is not to look for patient zero, the first person infected—or even a hypothetical bat zero, the single animal from which the novel virus jumped. It’s likely neither of those will ever be found. The goal instead is to elucidate the ecosystem—physical, but also viral—in which the spillover happened and ask what could make it likely to happen again.
“This is not a simple case of going to a market and picking up samples and testing,” says Peter Daszak, the president of the nonprofit research organization EcoHealth Alliance, who leads the Lancet commission task force. “This is about what's been changing on the ground in the region, in terms of the ecology of viruses and the social science of contact with wildlife—going back to SARS—and asking what research was done that could have been used to protect us, and was done or was not.”
The effort isn’t going to resemble movie-style moonsuit-clad narratives of disease detection, Daszak says—not least because, as of now, the teams still can’t travel to China. And, intellectually, it won’t proceed like them, either.
“There's a disconnect between what the public thinks goes on in missions like this and what can be done,” he says. “There’s an expectation of holding up a magnifying glass and finding the smoking gun, a criminal law approach. But we're never going to be beyond a reasonable doubt with the origins of Covid. Science doesn't work like that. Science works on the civil law approach: Where does the preponderance of evidence fit?”
“The team will review the global literature comprehensively and from multiple perspectives (ecology, virology, public health practices), and will do its best to engage with China’s public health leaders and scientists,” he said. “The team will also invite inputs from those who would like to submit information or who have advanced particular theories or possibilities about the origins of SARS-CoV-2.”
The obstacles are formidable, and that’s before the teams tangle with the politics of the disease. The Trump administration, of course, has made the pandemic political, persistently calling the novel coronavirus the “China virus.” In the spring, President Donald Trump and Secretary of State Mike Pompeo pushed theories that the virus was the result of a Wuhan lab accident and possibly human-made. (The US intelligence community rebuked that theory in April.) Despite that refutation—and protests from the WHO and from Asian Americans—the White House continued to stigmatize China’s connection to the virus’s emergence. In fact, a peer-reviewed $3.7 million National Institutes of Health grant to Daszak’s organization was taken away last April after the White House discovered that EcoHealth Alliance collaborates with the Wuhan Institute of Virology.
But the government of China has also used scientific findings for political purposes. Chinese scientists recently aired theories that the virus was imported into the country in frozen food, and also that it was circulating on other continents months before appearing in Wuhan. And it’s not at all clear that China will allow either team in. The WHO effort has been in planning stages since February; in October, Michael Ryan, executive director of its health emergencies program, disclosed that the mission will have two phases, one conducted in China by Chinese researchers and a second involving the multinational team. The Lancet effort could equally be hobbled by politics or by the restrictions on movement imposed by the pandemic itself.
Meanwhile, new pieces of research keep suggesting reframings of the origin story. On Monday, scientists at the US Centers for Disease Control and Prevention wrote in Clinical Infectious Diseases that they have identified antibodies to SARS CoV-2 in samples of donated blood collected on the US West Coast from December 13–16, 2019. If their observation is correct, it would mean the virus was present in the US as the first cases were being found in China, and a full month before the first known US case. Researchers in France have found molecular evidence of the virus in a sputum sample taken from a man hospitalized on December 27, 2019. And Italian scientists recently announced they have found antibodies in blood samples taken during lung-cancer screenings as far back as September 2019.
Still, the earliest confirmed human cases remain the first clusters identified in Wuhan in mid-December 2019, which the email list ProMED revealed to the world on December 30. The strongest animal associations remain coronaviruses found in bats in Yunnan, several provinces away, in 2013. The genetic sequence of that virus, which was collected from a live bat, was 96 percent similar to the viruses found in Wuhan residents in 2019. Those 4 percentage points of difference are enough to indicate that the bat virus had not simply passed to humans a single time; it might have undergone multiple crossings from bats into humans and then to other humans, or from bats to other animals and on into humans from there. Despite some provocative research—the association of many of the first human cases with a Wuhan seafood market that also sold wild animals; an identification of a similar virus in pangolins; recent finds of of the SARS-CoV-2 virus in minks in Europe and the US—no clear candidate for that evolutionary rest stop has emerged.
It is possible that none will. It turns out that the outbreaks in which scientists have traced a provable animal spillover from a host species into an intermediate animal and then into humans are rare bright spots in the hard slog of wildlife ecology, and not at all routine. The classic example—and the basis for the movie Contagion—is the emergence of Nipah virus in Malaysia in 1998. Clearing forests for farmland pushed a bat species to the forest edge, where pig farmers had set up; bats settled in trees over the pig pens and contaminated the swill the pigs were fed; pigs got sick and were culled; humans picked up the infection from their pigs, then died. When epidemiologists investigated the outbreak, all of the pieces were visible at once: the farms, the pigs, a nearby cave where the bats roosted. They found similar viral sequences in all of them.
But that Malaysia outbreak, which seemed paradigmatic, was an outlier. There has not been another Nipah outbreak in Malaysia since. Since 2000, though, there have been near-annual outbreaks of Nipah on the border of India and Bangladesh, occurring among families whose livelihood is collecting date-palm juice. As in the first outbreak, bats are the source of the virus. But unlike that first outbreak, the infection has passed directly from bats to humans. Pigs have never been involved again.
Ebola—which may be the most-studied zoonotic infection and also originates in bats—is similarly resistant to prediction. Researchers showed in 2016 that most of the 34 spillovers of Ebolavirus into humans that had occurred up to that point would not have been predicted by the best models that had been achieved. They came as a surprise.
“When we talk about spillovers, we are usually talking about weird outbreaks, because it’s the weird ones we end up knowing the most about,” says Colin J. Carlson, a global change biologist, assistant professor at Georgetown University Medical Center, and principal investigator of a consortium called the Viral Emergence Research Initiative. “Most of the time we don’t know what’s going on, as a default, because we don’t have surveillance systems that are operating at the level we need.”
The first SARS outbreak, which began in late 2002 in China and migrated to 30 countries before being extinguished in mid-2003, was also an outlier—not necessarily because of the animal species involved, though those relationships were complex, but because of the speed with which its cause was pieced together. “The thing that made SARS I easier was that there was a clear epidemiological link: A lot of the early cases were people who were animal handlers who were associated with wet markets in Guangdong, and that led to civet cats and raccoon dogs,” says David Wang, a professor of pathology and immunology at Washington University in St. Louis who helped characterize the virus behind that outbreak in May 2003, only two months after it was identified and three months after the disease broke out from Hong Kong to circle the world. “Later it became clear that the civet cats did not have the infection until they got into the wet market, so they must have acquired it from some other animal. And that led people to think about all the candidate animals, and that led to bats.”
Another way to say that is: The association of the first SARS coronavirus and its animal host benefited from luck. There was a quick alert, a quick identification, a quick, coordinated international response. None of those factors may be present now.
“Honestly, I think it's going to be very challenging [to identify the origin of SARS-CoV-2], because we are so far removed from the timing of the first cases,” Wang says. If an intermediate host species exists, he points out, the virus might have occupied those animals only transiently, and it may not be detectable now. Or the intermediate hosts might not have been present in the Wuhan market at all; the virus might have migrated into humans somewhere in the vast rest of China. After all, the Yunnan bat virus with the 96 percent homology to the Wuhan human strain was found approximately 1,000 miles away.
These possibilities make searching for the origin of the novel coronavirus a complicated proposition. “These things typically take a really long time, longer than they should,” says Christine Kreuder Johnson, a professor and director of the EpiCenter for Disease Dynamics at UC Davis and former director of Predict, a federal disease-detection program defunded under the Trump administration. (Zoonotic disease ecology is a small field: Johnson, Wang, and Daszak all direct centers within a new NIH research network that was set up in September.)
“The task force will have to look at evidence of earlier spillover in human populations—archived specimens that have been stored—and that would need to be done throughout even rural areas of China, never mind the actual site in Wuhan,” she says. “Testing animals throughout that range would also be very insightful, because we’ll need to find a virus much more closely related than the one the community has in hand. And then the last piece to put together the causal pathway is, what were the human activities that then brought the virus into the human population?”
If all those conditions can be fulfilled, it still will accomplish only half of what the teams intend to do: the part about finding where SARS-CoV-2 came from. The second task is preventing whatever emerges next. That is yet more complex, and researchers disagree about the tactics to choose and the strategies to implement them. In past projects, Daszak and Johnson have argued for more intense detection of novel viruses in wildlife, to find them before the pathogens make the zoonotic jump. But Carlson points out that global health has detected such viruses in the past without adequately preparing for their impact: Zika virus, which caused more than 1 million cases of illness and birth defects in the Americas from 2015 through 2018, was first collected from a monkey in 1947.
“We’re already at the stage where we've discovered the viruses, and we’ve noticed them spilling over,” Carlson says. “What we can try to do is to get to a point where we're detecting those outbreaks as early as possible, and there's no way for that to not fall on health care systems.”
It’s a big ask. Intensified detection in humans might require doing a thorough evaluation of any patient who comes to any hospital or clinic with the kind of symptoms that are usually dismissed: an unexplained fever or the symptoms of a respiratory infection that has no obvious cause. Then it would require putting that report into some sort of global alert registry, so that any emerging pattern can be perceived before the illness spirals beyond containment. There is no such early-warning system. In September, two Oxford researchers wrote: “Without it, we are flying blind.”
The last piece of the effort to detect the origin of Covid, in other words, might further burden the hospitals now being devastated by the pandemic. To create change in its wake, it won’t be enough to devise detection systems that ring international alarms when a novel virus is found in wildlife. It will be to ring an alarm that echoes globally as soon as a novel virus is identified in a human—and that may be even harder to achieve.
https://www.wired.com/story/two-global- ... obal-en-GB
With cases soaring in the United States and elsewhere, the Covid-19 pandemic is nowhere near its end
With three vaccines reporting trial data and two apparently nearing approval by the US Food and Drug Administration, it may be reaching a pivot point. In what feels like a moment of drawing breath and taking stock, international researchers are turning their attention from the present back to the start of the pandemic, aiming to untangle its origin and asking what lessons can be learned to keep this from happening again.
Two efforts are happening in parallel. On November 5, the World Health Organization quietly published the rules of engagement for a long-planned and months-delayed mission that creates a multinational team of researchers who will pursue how the virus leaped species. Meanwhile, last week, a commission created by The Lancet and headed by the economist and policy expert Jeffrey Sachs announced the formation of its own international effort, a task force of 12 experts from nine countries who will undertake similar tasks.
Both groups will face the same complex problems. It has been approximately a year since the first cases of a pneumonia of unknown origin appeared in Wuhan, China, and about 11 months since the pneumonia’s cause was identified as a novel coronavirus, probably originating in bats. The experts will have to retrace a chain of transmission—one or multiple leaps of the virus from the animal world into humans—using interviews, stored biological samples, lab assays, environmental surveys, genomic data, and the thousands of papers published since the pandemic began, all while following a trail that may have gone cold.
The point is not to look for patient zero, the first person infected—or even a hypothetical bat zero, the single animal from which the novel virus jumped. It’s likely neither of those will ever be found. The goal instead is to elucidate the ecosystem—physical, but also viral—in which the spillover happened and ask what could make it likely to happen again.
“This is not a simple case of going to a market and picking up samples and testing,” says Peter Daszak, the president of the nonprofit research organization EcoHealth Alliance, who leads the Lancet commission task force. “This is about what's been changing on the ground in the region, in terms of the ecology of viruses and the social science of contact with wildlife—going back to SARS—and asking what research was done that could have been used to protect us, and was done or was not.”
The effort isn’t going to resemble movie-style moonsuit-clad narratives of disease detection, Daszak says—not least because, as of now, the teams still can’t travel to China. And, intellectually, it won’t proceed like them, either.
“There's a disconnect between what the public thinks goes on in missions like this and what can be done,” he says. “There’s an expectation of holding up a magnifying glass and finding the smoking gun, a criminal law approach. But we're never going to be beyond a reasonable doubt with the origins of Covid. Science doesn't work like that. Science works on the civil law approach: Where does the preponderance of evidence fit?”
“The team will review the global literature comprehensively and from multiple perspectives (ecology, virology, public health practices), and will do its best to engage with China’s public health leaders and scientists,” he said. “The team will also invite inputs from those who would like to submit information or who have advanced particular theories or possibilities about the origins of SARS-CoV-2.”
The obstacles are formidable, and that’s before the teams tangle with the politics of the disease. The Trump administration, of course, has made the pandemic political, persistently calling the novel coronavirus the “China virus.” In the spring, President Donald Trump and Secretary of State Mike Pompeo pushed theories that the virus was the result of a Wuhan lab accident and possibly human-made. (The US intelligence community rebuked that theory in April.) Despite that refutation—and protests from the WHO and from Asian Americans—the White House continued to stigmatize China’s connection to the virus’s emergence. In fact, a peer-reviewed $3.7 million National Institutes of Health grant to Daszak’s organization was taken away last April after the White House discovered that EcoHealth Alliance collaborates with the Wuhan Institute of Virology.
But the government of China has also used scientific findings for political purposes. Chinese scientists recently aired theories that the virus was imported into the country in frozen food, and also that it was circulating on other continents months before appearing in Wuhan. And it’s not at all clear that China will allow either team in. The WHO effort has been in planning stages since February; in October, Michael Ryan, executive director of its health emergencies program, disclosed that the mission will have two phases, one conducted in China by Chinese researchers and a second involving the multinational team. The Lancet effort could equally be hobbled by politics or by the restrictions on movement imposed by the pandemic itself.
Meanwhile, new pieces of research keep suggesting reframings of the origin story. On Monday, scientists at the US Centers for Disease Control and Prevention wrote in Clinical Infectious Diseases that they have identified antibodies to SARS CoV-2 in samples of donated blood collected on the US West Coast from December 13–16, 2019. If their observation is correct, it would mean the virus was present in the US as the first cases were being found in China, and a full month before the first known US case. Researchers in France have found molecular evidence of the virus in a sputum sample taken from a man hospitalized on December 27, 2019. And Italian scientists recently announced they have found antibodies in blood samples taken during lung-cancer screenings as far back as September 2019.
Still, the earliest confirmed human cases remain the first clusters identified in Wuhan in mid-December 2019, which the email list ProMED revealed to the world on December 30. The strongest animal associations remain coronaviruses found in bats in Yunnan, several provinces away, in 2013. The genetic sequence of that virus, which was collected from a live bat, was 96 percent similar to the viruses found in Wuhan residents in 2019. Those 4 percentage points of difference are enough to indicate that the bat virus had not simply passed to humans a single time; it might have undergone multiple crossings from bats into humans and then to other humans, or from bats to other animals and on into humans from there. Despite some provocative research—the association of many of the first human cases with a Wuhan seafood market that also sold wild animals; an identification of a similar virus in pangolins; recent finds of of the SARS-CoV-2 virus in minks in Europe and the US—no clear candidate for that evolutionary rest stop has emerged.
It is possible that none will. It turns out that the outbreaks in which scientists have traced a provable animal spillover from a host species into an intermediate animal and then into humans are rare bright spots in the hard slog of wildlife ecology, and not at all routine. The classic example—and the basis for the movie Contagion—is the emergence of Nipah virus in Malaysia in 1998. Clearing forests for farmland pushed a bat species to the forest edge, where pig farmers had set up; bats settled in trees over the pig pens and contaminated the swill the pigs were fed; pigs got sick and were culled; humans picked up the infection from their pigs, then died. When epidemiologists investigated the outbreak, all of the pieces were visible at once: the farms, the pigs, a nearby cave where the bats roosted. They found similar viral sequences in all of them.
But that Malaysia outbreak, which seemed paradigmatic, was an outlier. There has not been another Nipah outbreak in Malaysia since. Since 2000, though, there have been near-annual outbreaks of Nipah on the border of India and Bangladesh, occurring among families whose livelihood is collecting date-palm juice. As in the first outbreak, bats are the source of the virus. But unlike that first outbreak, the infection has passed directly from bats to humans. Pigs have never been involved again.
Ebola—which may be the most-studied zoonotic infection and also originates in bats—is similarly resistant to prediction. Researchers showed in 2016 that most of the 34 spillovers of Ebolavirus into humans that had occurred up to that point would not have been predicted by the best models that had been achieved. They came as a surprise.
“When we talk about spillovers, we are usually talking about weird outbreaks, because it’s the weird ones we end up knowing the most about,” says Colin J. Carlson, a global change biologist, assistant professor at Georgetown University Medical Center, and principal investigator of a consortium called the Viral Emergence Research Initiative. “Most of the time we don’t know what’s going on, as a default, because we don’t have surveillance systems that are operating at the level we need.”
The first SARS outbreak, which began in late 2002 in China and migrated to 30 countries before being extinguished in mid-2003, was also an outlier—not necessarily because of the animal species involved, though those relationships were complex, but because of the speed with which its cause was pieced together. “The thing that made SARS I easier was that there was a clear epidemiological link: A lot of the early cases were people who were animal handlers who were associated with wet markets in Guangdong, and that led to civet cats and raccoon dogs,” says David Wang, a professor of pathology and immunology at Washington University in St. Louis who helped characterize the virus behind that outbreak in May 2003, only two months after it was identified and three months after the disease broke out from Hong Kong to circle the world. “Later it became clear that the civet cats did not have the infection until they got into the wet market, so they must have acquired it from some other animal. And that led people to think about all the candidate animals, and that led to bats.”
Another way to say that is: The association of the first SARS coronavirus and its animal host benefited from luck. There was a quick alert, a quick identification, a quick, coordinated international response. None of those factors may be present now.
“Honestly, I think it's going to be very challenging [to identify the origin of SARS-CoV-2], because we are so far removed from the timing of the first cases,” Wang says. If an intermediate host species exists, he points out, the virus might have occupied those animals only transiently, and it may not be detectable now. Or the intermediate hosts might not have been present in the Wuhan market at all; the virus might have migrated into humans somewhere in the vast rest of China. After all, the Yunnan bat virus with the 96 percent homology to the Wuhan human strain was found approximately 1,000 miles away.
These possibilities make searching for the origin of the novel coronavirus a complicated proposition. “These things typically take a really long time, longer than they should,” says Christine Kreuder Johnson, a professor and director of the EpiCenter for Disease Dynamics at UC Davis and former director of Predict, a federal disease-detection program defunded under the Trump administration. (Zoonotic disease ecology is a small field: Johnson, Wang, and Daszak all direct centers within a new NIH research network that was set up in September.)
“The task force will have to look at evidence of earlier spillover in human populations—archived specimens that have been stored—and that would need to be done throughout even rural areas of China, never mind the actual site in Wuhan,” she says. “Testing animals throughout that range would also be very insightful, because we’ll need to find a virus much more closely related than the one the community has in hand. And then the last piece to put together the causal pathway is, what were the human activities that then brought the virus into the human population?”
If all those conditions can be fulfilled, it still will accomplish only half of what the teams intend to do: the part about finding where SARS-CoV-2 came from. The second task is preventing whatever emerges next. That is yet more complex, and researchers disagree about the tactics to choose and the strategies to implement them. In past projects, Daszak and Johnson have argued for more intense detection of novel viruses in wildlife, to find them before the pathogens make the zoonotic jump. But Carlson points out that global health has detected such viruses in the past without adequately preparing for their impact: Zika virus, which caused more than 1 million cases of illness and birth defects in the Americas from 2015 through 2018, was first collected from a monkey in 1947.
“We’re already at the stage where we've discovered the viruses, and we’ve noticed them spilling over,” Carlson says. “What we can try to do is to get to a point where we're detecting those outbreaks as early as possible, and there's no way for that to not fall on health care systems.”
It’s a big ask. Intensified detection in humans might require doing a thorough evaluation of any patient who comes to any hospital or clinic with the kind of symptoms that are usually dismissed: an unexplained fever or the symptoms of a respiratory infection that has no obvious cause. Then it would require putting that report into some sort of global alert registry, so that any emerging pattern can be perceived before the illness spirals beyond containment. There is no such early-warning system. In September, two Oxford researchers wrote: “Without it, we are flying blind.”
The last piece of the effort to detect the origin of Covid, in other words, might further burden the hospitals now being devastated by the pandemic. To create change in its wake, it won’t be enough to devise detection systems that ring international alarms when a novel virus is found in wildlife. It will be to ring an alarm that echoes globally as soon as a novel virus is identified in a human—and that may be even harder to achieve.
https://www.wired.com/story/two-global- ... obal-en-GB
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Why some people get severely ill
Why some people with coronavirus have no symptoms and others get extremely ill is one of the pandemic's biggest puzzles
A study in Nature of more than 2,200 intensive care patients has identified specific genes that may hold the answer.
They make some people more susceptible to severe Covid-19 symptoms.
The findings shed light on where the immune system goes wrong, which could help identify new treatments.
These will continue to be needed even though vaccines are being developed, says Dr Kenneth Baillie, a consultant in medicine at the Royal Infirmary in Edinburgh, who led the Genomicc project.
"Vaccines should drastically decrease the numbers of covid cases, but it's likely doctors will still be treating the disease in intensive care for a number of years around the world, so there is an urgent need to find new treatments."
'Angry' cells
Scientists looked at the DNA of patients in more than 200 intensive care units in UK hospitals.
They scanned each person’s genes, which contain the instructions for every biological process - including how to fight a virus.
Their genomes were then compared with the DNA of healthy people to pinpoint any genetic differences, and a number were found - the first in a gene called TYK2.
“It is part of the system that makes your immune cells more angry, and more inflammatory,” explained Dr Baillie.
But if the gene is faulty, this immune response can go into overdrive, putting patients at risk of damaging lung inflammation.
A class of anti-inflammatory drugs already used for conditions such as rheumatoid arthritis targets this biological mechanism, including a drug called Baricitinib.
“It makes it a very plausible candidate for a new treatment,” Dr Baillie said. “But of course, we need to do large-scale clinical trials in order to find out if that's true or not.”
Too little interferon
Genetic differences were also found in a gene called DPP9, which plays a role in inflammation, and in a gene called OAS, which helps to stop the virus from making copies of itself.
Variations in a gene called IFNAR2 were also identified in the intensive care patients.
IFNAR2 is linked to a potent anti-viral molecule called interferon, which helps to kick-start the immune system as soon as an infection is detected.
It’s thought that producing too little interferon can give the virus an early advantage, allowing it to quickly replicate, leading to more severe disease.
Two other recent studies published in the journal Science have also implicated interferon in Covid cases, through both genetic mutations and an autoimmune disorder that affects its production.
Prof Jean-Laurent Casanova, who carried out the research, from The Rockefeller University in New York, said: “[Interferon] accounted for nearly 15% of the critical Covid-19 cases internationally enrolled in our cohort."
Interferon can be given as a treatment, but a World Health Organization clinical trial concluded that it did not help very sick patients. However, Prof Casanova said the timing was important.
He explained: “I hope that if given in the first two, three, four days of infection, the interferon would work, because it essentially would provide the molecule that the [patient] does not produce by himself or by herself.”
'When things go wrong'
Dr Vanessa Sancho-Shimizu, a geneticist from Imperial College London, said that the genetic discoveries were providing an unprecedented insight into the biology of the disease.
“It really is an example of precision medicine, where we can actually identify the moment at which things have gone awry in that individual,” she told BBC News.
“The findings from these genetic studies will help us identify particular molecular pathways that could be targets for therapeutic intervention," she said.
But the genome still holds some mysteries.
The Genomicc study - and several others - has revealed a cluster of genes on chromosome 3 strongly linked to severe symptoms. However, the biology underpinning this is not yet understood.
More patients will now be asked to take part in this research.
Dr Baillie said: “We need everyone, but we're particularly keen to recruit people from minority ethnic groups who are over-represented in the critically ill population."
He added: “There's still a very urgent need to find new treatments for this disease and we have to make the right choices about which treatments to try next, because we don't have time to make mistakes."
https://www.bbc.co.uk/news/health-54832563
Why some people with coronavirus have no symptoms and others get extremely ill is one of the pandemic's biggest puzzles
A study in Nature of more than 2,200 intensive care patients has identified specific genes that may hold the answer.
They make some people more susceptible to severe Covid-19 symptoms.
The findings shed light on where the immune system goes wrong, which could help identify new treatments.
These will continue to be needed even though vaccines are being developed, says Dr Kenneth Baillie, a consultant in medicine at the Royal Infirmary in Edinburgh, who led the Genomicc project.
"Vaccines should drastically decrease the numbers of covid cases, but it's likely doctors will still be treating the disease in intensive care for a number of years around the world, so there is an urgent need to find new treatments."
'Angry' cells
Scientists looked at the DNA of patients in more than 200 intensive care units in UK hospitals.
They scanned each person’s genes, which contain the instructions for every biological process - including how to fight a virus.
Their genomes were then compared with the DNA of healthy people to pinpoint any genetic differences, and a number were found - the first in a gene called TYK2.
“It is part of the system that makes your immune cells more angry, and more inflammatory,” explained Dr Baillie.
But if the gene is faulty, this immune response can go into overdrive, putting patients at risk of damaging lung inflammation.
A class of anti-inflammatory drugs already used for conditions such as rheumatoid arthritis targets this biological mechanism, including a drug called Baricitinib.
“It makes it a very plausible candidate for a new treatment,” Dr Baillie said. “But of course, we need to do large-scale clinical trials in order to find out if that's true or not.”
Too little interferon
Genetic differences were also found in a gene called DPP9, which plays a role in inflammation, and in a gene called OAS, which helps to stop the virus from making copies of itself.
Variations in a gene called IFNAR2 were also identified in the intensive care patients.
IFNAR2 is linked to a potent anti-viral molecule called interferon, which helps to kick-start the immune system as soon as an infection is detected.
It’s thought that producing too little interferon can give the virus an early advantage, allowing it to quickly replicate, leading to more severe disease.
Two other recent studies published in the journal Science have also implicated interferon in Covid cases, through both genetic mutations and an autoimmune disorder that affects its production.
Prof Jean-Laurent Casanova, who carried out the research, from The Rockefeller University in New York, said: “[Interferon] accounted for nearly 15% of the critical Covid-19 cases internationally enrolled in our cohort."
Interferon can be given as a treatment, but a World Health Organization clinical trial concluded that it did not help very sick patients. However, Prof Casanova said the timing was important.
He explained: “I hope that if given in the first two, three, four days of infection, the interferon would work, because it essentially would provide the molecule that the [patient] does not produce by himself or by herself.”
'When things go wrong'
Dr Vanessa Sancho-Shimizu, a geneticist from Imperial College London, said that the genetic discoveries were providing an unprecedented insight into the biology of the disease.
“It really is an example of precision medicine, where we can actually identify the moment at which things have gone awry in that individual,” she told BBC News.
“The findings from these genetic studies will help us identify particular molecular pathways that could be targets for therapeutic intervention," she said.
But the genome still holds some mysteries.
The Genomicc study - and several others - has revealed a cluster of genes on chromosome 3 strongly linked to severe symptoms. However, the biology underpinning this is not yet understood.
More patients will now be asked to take part in this research.
Dr Baillie said: “We need everyone, but we're particularly keen to recruit people from minority ethnic groups who are over-represented in the critically ill population."
He added: “There's still a very urgent need to find new treatments for this disease and we have to make the right choices about which treatments to try next, because we don't have time to make mistakes."
https://www.bbc.co.uk/news/health-54832563
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Russians refuse to take Sputnik V
Like the Cold War satellite of the same name, Russia hoped Sputnik V would be a world-beater. It was the first to be registered for emergency use although it had only been tested on a few dozen people
Now, after Britain became the first country in the West to administer a Covid-19 vaccine, Russians are also receiving a similar offer. It is part of a mass inoculation campaign ordered by President Putin, but Sputnik V is still in the midst of trials to check it's safe and actually works, leading to some Russians being wary of listening to their President.
In an independent poll by the Levada Centre, 59 percent of respondents said they would not get the vaccine, even if it was voluntary and free of charge.
She told Express.co.uk: “There are a couple of issues. If the trials are still ongoing then it is a cause for concern when they are rolling it out.
“We are lucky that, in the UK, we have the data there. We have the MHRA (Medicines and Healthcare products Regulatory Agency) who have reviewed the data for the Pfizer vaccine and have declared it okay to rollout.
“We’ve gone through the process, the regulatory process.”
Sputnik's developers claim it offers 95 percent protection against the coronavirus, placing it alongside developments in the US and Europe.
That conclusion was based on 39 infections among 18,794 study participants that received both doses of either the vaccine or the placebo.
Some Russians have chosen not to take the vaccine
It is a much lower number of infections than Western drugmakers looked at when assessing the effectiveness of their vaccines.
Svetlana Zavidova, whose organisation monitors clinical trials in Russia, would like to see further studies.
She said: "We hope the vaccine is effective, but it's difficult to trust some of the figures.
"We don't see the point of such a rush, other than announcing how we beat the rest of the world.
"I think there's a struggle between scientists and politicians, and the latter are winning."
Like other countries, Russia presents its trial results to an external panel of experts for analysis, but that process has not been fully completed yet.
Some believe the vaccine has been rushed out in Russia
Moreover, Putin faces more potential setbacks after boasting that tens of millions of doses of their vaccine would be rolling off conveyor belts this year.
It is looking more likely to be a fraction of that.
The Kremlin now expects a modest two million doses of Sputnik by the end of December.
The Russian vaccine comprises two entirely different injections - using different "vectors" or carriers for the coronavirus each time.
This process is said to make it more effective, but it also piles extra pressure on producers.
Putin hopes to have two million doses by the end of the month
https://www.express.co.uk/news/world/13 ... trials-spt
Like the Cold War satellite of the same name, Russia hoped Sputnik V would be a world-beater. It was the first to be registered for emergency use although it had only been tested on a few dozen people
Now, after Britain became the first country in the West to administer a Covid-19 vaccine, Russians are also receiving a similar offer. It is part of a mass inoculation campaign ordered by President Putin, but Sputnik V is still in the midst of trials to check it's safe and actually works, leading to some Russians being wary of listening to their President.
In an independent poll by the Levada Centre, 59 percent of respondents said they would not get the vaccine, even if it was voluntary and free of charge.
She told Express.co.uk: “There are a couple of issues. If the trials are still ongoing then it is a cause for concern when they are rolling it out.
“We are lucky that, in the UK, we have the data there. We have the MHRA (Medicines and Healthcare products Regulatory Agency) who have reviewed the data for the Pfizer vaccine and have declared it okay to rollout.
“We’ve gone through the process, the regulatory process.”
Sputnik's developers claim it offers 95 percent protection against the coronavirus, placing it alongside developments in the US and Europe.
That conclusion was based on 39 infections among 18,794 study participants that received both doses of either the vaccine or the placebo.
Some Russians have chosen not to take the vaccine
It is a much lower number of infections than Western drugmakers looked at when assessing the effectiveness of their vaccines.
Svetlana Zavidova, whose organisation monitors clinical trials in Russia, would like to see further studies.
She said: "We hope the vaccine is effective, but it's difficult to trust some of the figures.
"We don't see the point of such a rush, other than announcing how we beat the rest of the world.
"I think there's a struggle between scientists and politicians, and the latter are winning."
Like other countries, Russia presents its trial results to an external panel of experts for analysis, but that process has not been fully completed yet.
Some believe the vaccine has been rushed out in Russia
Moreover, Putin faces more potential setbacks after boasting that tens of millions of doses of their vaccine would be rolling off conveyor belts this year.
It is looking more likely to be a fraction of that.
The Kremlin now expects a modest two million doses of Sputnik by the end of December.
The Russian vaccine comprises two entirely different injections - using different "vectors" or carriers for the coronavirus each time.
This process is said to make it more effective, but it also piles extra pressure on producers.
Putin hopes to have two million doses by the end of the month
https://www.express.co.uk/news/world/13 ... trials-spt
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
Oxford-AstraZeneca vaccine
Bogus reports - accidental finds - the story of the jab
In the early hours of Saturday 11 January, Prof Teresa Lambe was woken up by the ping of her email. The information she had been waiting for had just arrived in her inbox: the genetic code for a new coronavirus, shared worldwide by scientists in China.
She got to work straight away, still in her pyjamas, and was glued to her laptop for the next 48 hours. "My family didn't see me very much that weekend, but I think that set the tone for the rest of the year," she says.
By Monday morning, she had it: the template for a new experimental coronavirus vaccine. The first death from the new virus was reported around the same time, but it was still a month before the disease it causes was named Covid-19.
Lambe's team at Oxford University's Jenner Institute, led by Prof Sarah Gilbert, was always on the lookout for Disease X - the name given to the unknown infectious agent that could trigger the next pandemic. They had already used their experimental vaccine system against malaria and flu and, crucially, against another type of coronavirus, Middle Eastern Respiratory Syndrome (Mers). So they were confident it could work again.
That weekend was the first step on a journey to create a vaccine at lightning speed, for a disease that would, in a matter of months, claim more than 1.5 million lives. I have been following the efforts of the Oxford scientists since the start. There have been dramas along the way, including:
"I went from someone who was aware of a small outbreak in China, which was of academic interest, to realising that it was going to change our lives. It was a chilling moment," Pollard says.
Pollard got in touch with Gilbert and found her team at the Jenner Institute was already working on a vaccine. They agreed to collaborate on a clinical trial.
By February the virus had spread to more than two dozen countries, including the UK and the US. There had been more than 1,000 deaths in China.
But getting funding for trials was still a struggle. It was all Gilbert thought about, getting up at 04:00 to "write the grant application trying to persuade somebody that money I'd been awarded for a different project would actually be better spent on this project," she says.
It was another month before the UK was put into lockdown, and the government announced funding to support clinical trials and manufacturing. This meant the vaccine could go into production.
The first doses were made at the university's own bio-manufacturing facility after Prof Catherine Green had created a cell culture from which it could be grown, using Lambe's blueprint.
The science behind the vaccine is fascinating. It is created from a modified virus that causes the common cold in chimpanzees, which has had a tiny bit of genetic material removed so that it can't trigger infection in humans. Into this gap is inserted a fragment of the genetic code for coronavirus. This forms the vaccine. The technical term is a viral vector vaccine.
Such vaccines are grown in human cell culture which has a gene added to allow the viral particles to multiply at an enormous rate. Billions and billions are grown in bioreactors, and then purified. One of the team said it was like the recipe for Coke - once you knew the secret, and followed a formula, it could be done anywhere. Having seen the procedure, with all the technicians, working on different parts of the recipe, I prefer to think of it as a bit like a Vaccine MasterChef.
Scientist at work in the lab
But lockdown brought significant challenges. Getting hold of PPE was a logistical nightmare, and Green was reduced to making hand sanitiser in the lab. Her team worked round the clock, doing double shifts, seven days a week.
"We didn't wait for the results of one stage before we went on to the next stage," she says. "So we took a risk... We still did the checks and if the checks had come back as a fail, we'd have had to throw away all the things we'd done."
The checks did not fail, and after seven weeks they had enough doses of the vaccine to start the first trial. By this stage 10,000 volunteers had been recruited - all of them signing up within a matter of hours. Elisa Granato, a microbiologist, and Edward O'Neill, a cancer researcher, were the first two to receive Oxford's trial jab in April.
For Lambe, the vaccination of the first individuals was a monumental step. "I remember walking home from the lab and almost breaking down because we had a vaccine and got it into somebody's arm," she says.
The team waited 48 hours to check Granato and O'Neill had had no serious adverse reactions. With both doing well, they immunised a further six volunteers, and then gradually ramped up the numbers.
media captionElisa Granato was the first volunteer to be injected
But only a few days later there were shocking reports on social media that the first volunteer, Granato, had died.
It wasn't true. I spoke to her that day, and she told me she had never felt better. I posted a bit of the interview online. It helped quash the story, but not completely. Bizarrely, there were suggestions the interview might be fake and she really needed to hold up that day's newspaper to the camera as "proof of life".
"It was very upsetting," says Gilbert. "A lot of work had to be done, for her sake as well as for the trial's sake, to make it clear that this was just fake news."
Disinformation spreads fast and has the potential to undo months of hard work. Green found it very stressful. "Oh God it's awful! To think how malicious that can be, how people are really spreading nasty things… that doesn't feel fair because my team were all doing their best," she says.
As the trial grew, it was clear that Oxford's small manufacturing facility would not be able to keep up with demand. The team decided to outsource some of the manufacturing to Italy. But when the first batch was ready, there was a snag - the Europe-wide lockdown meant there were no flights to airlift it from Rome.
"Eventually we chartered a plane to bring 500 doses of vaccine because it was the only way we could get it here in time," says Green.
This is a really important part of the story which ended up being highly significant months later.
The Italian manufacturers used a different technique to Oxford to check the concentration of the vaccine - effectively how many viral particles are floating in each dose. When the Oxford scientists used their method, it appeared that the Italian vaccine was double strength. What to do? Calls were made to the medical regulators. It was agreed that volunteers should be given a half measure of the vaccine, on the basis that it was likely to equate to something more like a regular dose. This was partly a safety issue - they preferred to give them too little rather than too much.
But after a week, the scientists became aware that something unusual was going on. The volunteers were getting none of the usual side-effects - such as sore arms or fever. About 1,300 volunteers had only received a half-dose of the vaccine, rather than a full one. The independent regulators said the trial should continue and that the half-dose group could remain in the study.
volunteer
The Oxford team bristle at any suggestion that there was a mistake, error, call it what you will. Perhaps the most accurate characterisation is that the volunteers were inadvertently given a lower dose. In months to come, they would be the stellar group in terms of vaccine efficacy.
From the start, the team at Oxford had had the goal of creating a vaccine that could help the world. To do that they would need billions of doses - something only industry could provide.
Sir Mene Pangalos, of the Cambridge-based pharmaceutical giant AstraZeneca, suggested the company could help.
But there was a potential hitch - Oxford insisted the vaccine should be affordable, which meant no profit for the pharmaceutical company. "Not usually the way big pharma works," says Pollard.
After intense negotiations, they reached a deal at the end of April. The vaccine would be provided on a not-for-profit basis worldwide, for the duration of the pandemic, and always at cost to low- and middle-income countries.
Most importantly of all, AstraZeneca agreed to take on the financial risk, even if the vaccine turned out not to work.
In May, in a huge vote of confidence, the UK government agreed to buy 100 million doses and provided nearly £90m in support.
By then there were nearly a dozen coronavirus vaccines in human trials around the world.
There's a mixture of anticipation, tinged with: Oh, I really hope it works!
At the start of summer, levels of coronavirus infection were falling in the UK. While this was good news for society, it made life more difficult for the trial, which relied on volunteers being exposed to coronavirus in order to figure out if the vaccine would protect them.
"There was a real risk that we wouldn't be able to get the answers that we'd hoped for really quickly," says Pollard.
They decided to expand to 19 sites around the UK.
For a vaccine that was intended for global use, it was important to test it in other countries too, to see how it worked in different populations. Trials began in South Africa and Brazil - which would soon have the second-highest death toll after the United States.
There is not one vaccine that can do the whole world, so the more the merrier
On 20 July, the initial results were released, showing whether the vaccine had stimulated the immune systems of the first 1,000 volunteers. The team was feeling the pressure.
"I've worked in vaccines for long enough to know that most vaccines actually don't work. And it's the worst feeling in the world to have such high expectations and then to see nothing," says Prof Katie Ewer, who leads the team studying T-cell response.
It was good news, though. The vaccine appeared to be safe, and had triggered the two-pronged immune response they were hoping for - producing antibodies which neutralise the virus, and T cells which can kill cells which have become infected. But the data also led to a change of strategy. Until then, the Oxford team had hoped to produce a vaccine that could be delivered in a single dose, so there was more vaccine to go round.
However, 10 participants who were given two doses showed a much stronger immune response, so all volunteers were invited back for a booster, to maximise the chances of protection.
Although these early results were promising, at this stage there was still no evidence to show whether the vaccine would actually protect against Covid-19.
How the coronavirus vaccine works: The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans. Scientists then added genes for the spike surface protein of the coronavirus. This should prompt the immune system to produce neutralising antibodies, which would recognise and prevent any future coronavirus infection.
What's more, up until this point it had only been tested on adults under the age of 55. Now older volunteers could be enrolled.
Unlike other vaccines being trialled, the Oxford team had been taking weekly swabs from all volunteers to check whether they were infected but showing no symptoms. If the vaccine could prevent silent transmission it could stop them from unwittingly passing on the virus. "That's a big deal. Because that means the virus could be stopped in its tracks," says Pollard.
Intriguingly, when the trial results came out, there were indications it may partly suppress transmission of the virus. But more evidence is needed.
By the end of the summer, trials of the vaccine were running in six countries including the UK, Brazil, South Africa and the United States. Nearly 20,000 volunteers had been recruited. But on 6 September suddenly everything stopped.
A participant had developed a rare neurological condition.
"In a clinical trial of tens of thousands of people, stuff happens," says Pollard. "People develop illnesses, there will be people who develop cancers and who develop neurological conditions - the difficulty... is to work out whether it's associated with the… vaccine.
An independent safety review did not find any reason to suspect the illness had been caused by the vaccine. But they couldn't rule it out either. The volunteer is still part of the trial and is recovering.
Safety regulators in the UK, Brazil and South Africa gave the green light for the trials to resume within days, but it was six weeks before they were allowed to resume in the US.
All this came amidst a climate of anti-vaccine sentiment - not just online, but with demonstrations taking place around the world.
Transparency and openness about the vaccination process is one way of overcoming such mistrust. This is partly why the extremely busy team of scientists has given the BBC access to their labs over the past year.
In November, two other vaccine trials published their results. They were astonishing. First Pfizer-BioNTech, then a week later Moderna, announced its vaccines were about 95% effective. The team in Oxford were encouraged.
Until then there was uncertainty that any vaccine could work on Covid-19, says Lambe. "There are so many infectious diseases out there that we can't impact."
Finally, on 21 November, the independent data safety committee was ready to reveal the Oxford-AstraZeneca findings. But the results were surprising - and more complex than expected.
Whereas Pfizer and Moderna had one efficacy figure from one big trial each, the Oxford jab ended up with three numbers: 70% overall, with the two full doses giving 62% protection while the smaller group, who were given that initial half dose had the highest protection, had 90%. It was a result no-one had expected.
Crucially, no-one who got the vaccine was hospitalised or got seriously ill due to Covid. Whereas in the control group there were 10 serious cases and one death.
The fact that those who had unwittingly been given the initial half dose from Italy showed stronger protection is intriguing. It may be caused by the immune system being primed more gradually, but the scientists can't yet explain it. Also, all the volunteers in this group were under 55.
Within days, concern was voiced. AstraZeneca accepts that the US regulator, the FDA is likely to hold off approval for now - the company is half-way through recruiting 30,000 volunteers for an American trial.
"We will take them through the data set but my assumption is that they will want to see the US data as well," says Mene Pangalos.
Here, the data is with the UK regulator - everyone is waiting for its decision.
Even at the lowest level of efficacy, the Oxford vaccine passes the effectiveness test with flying colours. The overall effectiveness of 70% is better than most flu vaccines.
Perhaps the main area of debate will be over older adults. There isn't much efficacy data yet from older age groups, since seniors were recruited later. This information will come. There is clear evidence however that the immune response to the vaccine does not decline with age, as it does against other vaccines like flu. This suggests, but doesn't prove, that the jab will offer protection in the elderly.
"Look, I think there is no doubt that we would've run the study a little bit differently if we'd been doing it from scratch, but ultimately we've done as good a job as we possibly can to translate that in to the data set that we can provide to the regulators around the world for approval," says Pangalos.
"The processes we've gone through are the same and just as rigorous as in normal times."
While the Pfizer vaccine is already being rolled out, the Oxford-AstraZeneca one has a crucial advantage - its vials can be stored and transported at normal fridge temperature.
This is game-changing, says Sir Jeremy Farrar, the head of Wellcome Trust. "If you have to store vaccines on dry ice that brings additional challenges of how to get it into villages in the UK, let alone villages in sub-Saharan Africa and Brazil."
The UK has preordered millions of vaccine doses which, together, will be more than enough to give every adult in the UK two doses next year. Four million doses of the Oxford vaccine are ready to be handed over to the NHS.
Numbers of orders/pre-orders
How does it feel to have got this far in a little under 12 months?
"It's a relief at this stage, says Gilbert, "that all of the work that people have done during the year and all of the money that's been spent on manufacturing large amounts of this vaccine, is not going to go to waste."
It was worth it! Because we worked really hard. I think we all had a little cry
On the day the results were made public, Green came home to find a colleague had left a magnum of English sparkling wine on her doorstep. But she hasn't had a chance to get together with enough people to drink it yet.
"It's a bit overwhelming to feel that the sacrifices we've made paid off and we did something big. It was worth it, because we worked really hard, I think we all had a little cry," says Green.
Pangalos hopes the vaccine means he will be able to visit his mother in Greece - they haven't seen each other since last Christmas.
The other members of the team haven't seen much of their families either, even when living in the same house. "My son said something the other day that really impacted me," says Lambe. "I was apologising for being late home from work again and he said, 'It's OK mummy, you're doing it for everybody. It's not just me you need to look after.' And that did bring a tear to my eye."
Over the course of the year it's been an emotional journey for everyone working on the vaccine,
But in the end it was the cold, hard science that really made the difference.
"I think science has been the exit strategy for this pandemic and vaccines are at the heart of that scientific endeavour," says Farrar. "It's been remarkable, there's never been a year of progress scientifically like this in my lifetime."
https://www.bbc.co.uk/news/health-55308216
Bogus reports - accidental finds - the story of the jab
In the early hours of Saturday 11 January, Prof Teresa Lambe was woken up by the ping of her email. The information she had been waiting for had just arrived in her inbox: the genetic code for a new coronavirus, shared worldwide by scientists in China.
She got to work straight away, still in her pyjamas, and was glued to her laptop for the next 48 hours. "My family didn't see me very much that weekend, but I think that set the tone for the rest of the year," she says.
By Monday morning, she had it: the template for a new experimental coronavirus vaccine. The first death from the new virus was reported around the same time, but it was still a month before the disease it causes was named Covid-19.
Lambe's team at Oxford University's Jenner Institute, led by Prof Sarah Gilbert, was always on the lookout for Disease X - the name given to the unknown infectious agent that could trigger the next pandemic. They had already used their experimental vaccine system against malaria and flu and, crucially, against another type of coronavirus, Middle Eastern Respiratory Syndrome (Mers). So they were confident it could work again.
That weekend was the first step on a journey to create a vaccine at lightning speed, for a disease that would, in a matter of months, claim more than 1.5 million lives. I have been following the efforts of the Oxford scientists since the start. There have been dramas along the way, including:
- a rush to charter a jet when a flight-ban prevented vaccine from getting into the country
dismay at totally false reports on social media that the first volunteer to be immunised had died
concern that falling infection rates over the summer would jeopardise the hope of quick results
how an initial half-dose of the vaccine unexpectedly provided the best protection
an admission from the chief of Oxford's partner, drug company AstraZeneca, that it would have run the trials "a bit differently".
"I went from someone who was aware of a small outbreak in China, which was of academic interest, to realising that it was going to change our lives. It was a chilling moment," Pollard says.
Pollard got in touch with Gilbert and found her team at the Jenner Institute was already working on a vaccine. They agreed to collaborate on a clinical trial.
By February the virus had spread to more than two dozen countries, including the UK and the US. There had been more than 1,000 deaths in China.
But getting funding for trials was still a struggle. It was all Gilbert thought about, getting up at 04:00 to "write the grant application trying to persuade somebody that money I'd been awarded for a different project would actually be better spent on this project," she says.
It was another month before the UK was put into lockdown, and the government announced funding to support clinical trials and manufacturing. This meant the vaccine could go into production.
The first doses were made at the university's own bio-manufacturing facility after Prof Catherine Green had created a cell culture from which it could be grown, using Lambe's blueprint.
The science behind the vaccine is fascinating. It is created from a modified virus that causes the common cold in chimpanzees, which has had a tiny bit of genetic material removed so that it can't trigger infection in humans. Into this gap is inserted a fragment of the genetic code for coronavirus. This forms the vaccine. The technical term is a viral vector vaccine.
Such vaccines are grown in human cell culture which has a gene added to allow the viral particles to multiply at an enormous rate. Billions and billions are grown in bioreactors, and then purified. One of the team said it was like the recipe for Coke - once you knew the secret, and followed a formula, it could be done anywhere. Having seen the procedure, with all the technicians, working on different parts of the recipe, I prefer to think of it as a bit like a Vaccine MasterChef.
Scientist at work in the lab
But lockdown brought significant challenges. Getting hold of PPE was a logistical nightmare, and Green was reduced to making hand sanitiser in the lab. Her team worked round the clock, doing double shifts, seven days a week.
"We didn't wait for the results of one stage before we went on to the next stage," she says. "So we took a risk... We still did the checks and if the checks had come back as a fail, we'd have had to throw away all the things we'd done."
The checks did not fail, and after seven weeks they had enough doses of the vaccine to start the first trial. By this stage 10,000 volunteers had been recruited - all of them signing up within a matter of hours. Elisa Granato, a microbiologist, and Edward O'Neill, a cancer researcher, were the first two to receive Oxford's trial jab in April.
For Lambe, the vaccination of the first individuals was a monumental step. "I remember walking home from the lab and almost breaking down because we had a vaccine and got it into somebody's arm," she says.
The team waited 48 hours to check Granato and O'Neill had had no serious adverse reactions. With both doing well, they immunised a further six volunteers, and then gradually ramped up the numbers.
media captionElisa Granato was the first volunteer to be injected
But only a few days later there were shocking reports on social media that the first volunteer, Granato, had died.
It wasn't true. I spoke to her that day, and she told me she had never felt better. I posted a bit of the interview online. It helped quash the story, but not completely. Bizarrely, there were suggestions the interview might be fake and she really needed to hold up that day's newspaper to the camera as "proof of life".
"It was very upsetting," says Gilbert. "A lot of work had to be done, for her sake as well as for the trial's sake, to make it clear that this was just fake news."
Disinformation spreads fast and has the potential to undo months of hard work. Green found it very stressful. "Oh God it's awful! To think how malicious that can be, how people are really spreading nasty things… that doesn't feel fair because my team were all doing their best," she says.
As the trial grew, it was clear that Oxford's small manufacturing facility would not be able to keep up with demand. The team decided to outsource some of the manufacturing to Italy. But when the first batch was ready, there was a snag - the Europe-wide lockdown meant there were no flights to airlift it from Rome.
"Eventually we chartered a plane to bring 500 doses of vaccine because it was the only way we could get it here in time," says Green.
This is a really important part of the story which ended up being highly significant months later.
The Italian manufacturers used a different technique to Oxford to check the concentration of the vaccine - effectively how many viral particles are floating in each dose. When the Oxford scientists used their method, it appeared that the Italian vaccine was double strength. What to do? Calls were made to the medical regulators. It was agreed that volunteers should be given a half measure of the vaccine, on the basis that it was likely to equate to something more like a regular dose. This was partly a safety issue - they preferred to give them too little rather than too much.
But after a week, the scientists became aware that something unusual was going on. The volunteers were getting none of the usual side-effects - such as sore arms or fever. About 1,300 volunteers had only received a half-dose of the vaccine, rather than a full one. The independent regulators said the trial should continue and that the half-dose group could remain in the study.
volunteer
The Oxford team bristle at any suggestion that there was a mistake, error, call it what you will. Perhaps the most accurate characterisation is that the volunteers were inadvertently given a lower dose. In months to come, they would be the stellar group in terms of vaccine efficacy.
From the start, the team at Oxford had had the goal of creating a vaccine that could help the world. To do that they would need billions of doses - something only industry could provide.
Sir Mene Pangalos, of the Cambridge-based pharmaceutical giant AstraZeneca, suggested the company could help.
But there was a potential hitch - Oxford insisted the vaccine should be affordable, which meant no profit for the pharmaceutical company. "Not usually the way big pharma works," says Pollard.
After intense negotiations, they reached a deal at the end of April. The vaccine would be provided on a not-for-profit basis worldwide, for the duration of the pandemic, and always at cost to low- and middle-income countries.
Most importantly of all, AstraZeneca agreed to take on the financial risk, even if the vaccine turned out not to work.
In May, in a huge vote of confidence, the UK government agreed to buy 100 million doses and provided nearly £90m in support.
By then there were nearly a dozen coronavirus vaccines in human trials around the world.
There's a mixture of anticipation, tinged with: Oh, I really hope it works!
At the start of summer, levels of coronavirus infection were falling in the UK. While this was good news for society, it made life more difficult for the trial, which relied on volunteers being exposed to coronavirus in order to figure out if the vaccine would protect them.
"There was a real risk that we wouldn't be able to get the answers that we'd hoped for really quickly," says Pollard.
They decided to expand to 19 sites around the UK.
For a vaccine that was intended for global use, it was important to test it in other countries too, to see how it worked in different populations. Trials began in South Africa and Brazil - which would soon have the second-highest death toll after the United States.
There is not one vaccine that can do the whole world, so the more the merrier
On 20 July, the initial results were released, showing whether the vaccine had stimulated the immune systems of the first 1,000 volunteers. The team was feeling the pressure.
"I've worked in vaccines for long enough to know that most vaccines actually don't work. And it's the worst feeling in the world to have such high expectations and then to see nothing," says Prof Katie Ewer, who leads the team studying T-cell response.
It was good news, though. The vaccine appeared to be safe, and had triggered the two-pronged immune response they were hoping for - producing antibodies which neutralise the virus, and T cells which can kill cells which have become infected. But the data also led to a change of strategy. Until then, the Oxford team had hoped to produce a vaccine that could be delivered in a single dose, so there was more vaccine to go round.
However, 10 participants who were given two doses showed a much stronger immune response, so all volunteers were invited back for a booster, to maximise the chances of protection.
Although these early results were promising, at this stage there was still no evidence to show whether the vaccine would actually protect against Covid-19.
How the coronavirus vaccine works: The vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees that has been modified so it cannot grow in humans. Scientists then added genes for the spike surface protein of the coronavirus. This should prompt the immune system to produce neutralising antibodies, which would recognise and prevent any future coronavirus infection.
What's more, up until this point it had only been tested on adults under the age of 55. Now older volunteers could be enrolled.
Unlike other vaccines being trialled, the Oxford team had been taking weekly swabs from all volunteers to check whether they were infected but showing no symptoms. If the vaccine could prevent silent transmission it could stop them from unwittingly passing on the virus. "That's a big deal. Because that means the virus could be stopped in its tracks," says Pollard.
Intriguingly, when the trial results came out, there were indications it may partly suppress transmission of the virus. But more evidence is needed.
By the end of the summer, trials of the vaccine were running in six countries including the UK, Brazil, South Africa and the United States. Nearly 20,000 volunteers had been recruited. But on 6 September suddenly everything stopped.
A participant had developed a rare neurological condition.
"In a clinical trial of tens of thousands of people, stuff happens," says Pollard. "People develop illnesses, there will be people who develop cancers and who develop neurological conditions - the difficulty... is to work out whether it's associated with the… vaccine.
An independent safety review did not find any reason to suspect the illness had been caused by the vaccine. But they couldn't rule it out either. The volunteer is still part of the trial and is recovering.
Safety regulators in the UK, Brazil and South Africa gave the green light for the trials to resume within days, but it was six weeks before they were allowed to resume in the US.
All this came amidst a climate of anti-vaccine sentiment - not just online, but with demonstrations taking place around the world.
Transparency and openness about the vaccination process is one way of overcoming such mistrust. This is partly why the extremely busy team of scientists has given the BBC access to their labs over the past year.
In November, two other vaccine trials published their results. They were astonishing. First Pfizer-BioNTech, then a week later Moderna, announced its vaccines were about 95% effective. The team in Oxford were encouraged.
Until then there was uncertainty that any vaccine could work on Covid-19, says Lambe. "There are so many infectious diseases out there that we can't impact."
Finally, on 21 November, the independent data safety committee was ready to reveal the Oxford-AstraZeneca findings. But the results were surprising - and more complex than expected.
Whereas Pfizer and Moderna had one efficacy figure from one big trial each, the Oxford jab ended up with three numbers: 70% overall, with the two full doses giving 62% protection while the smaller group, who were given that initial half dose had the highest protection, had 90%. It was a result no-one had expected.
Crucially, no-one who got the vaccine was hospitalised or got seriously ill due to Covid. Whereas in the control group there were 10 serious cases and one death.
The fact that those who had unwittingly been given the initial half dose from Italy showed stronger protection is intriguing. It may be caused by the immune system being primed more gradually, but the scientists can't yet explain it. Also, all the volunteers in this group were under 55.
Within days, concern was voiced. AstraZeneca accepts that the US regulator, the FDA is likely to hold off approval for now - the company is half-way through recruiting 30,000 volunteers for an American trial.
"We will take them through the data set but my assumption is that they will want to see the US data as well," says Mene Pangalos.
Here, the data is with the UK regulator - everyone is waiting for its decision.
Even at the lowest level of efficacy, the Oxford vaccine passes the effectiveness test with flying colours. The overall effectiveness of 70% is better than most flu vaccines.
Perhaps the main area of debate will be over older adults. There isn't much efficacy data yet from older age groups, since seniors were recruited later. This information will come. There is clear evidence however that the immune response to the vaccine does not decline with age, as it does against other vaccines like flu. This suggests, but doesn't prove, that the jab will offer protection in the elderly.
"Look, I think there is no doubt that we would've run the study a little bit differently if we'd been doing it from scratch, but ultimately we've done as good a job as we possibly can to translate that in to the data set that we can provide to the regulators around the world for approval," says Pangalos.
"The processes we've gone through are the same and just as rigorous as in normal times."
While the Pfizer vaccine is already being rolled out, the Oxford-AstraZeneca one has a crucial advantage - its vials can be stored and transported at normal fridge temperature.
This is game-changing, says Sir Jeremy Farrar, the head of Wellcome Trust. "If you have to store vaccines on dry ice that brings additional challenges of how to get it into villages in the UK, let alone villages in sub-Saharan Africa and Brazil."
The UK has preordered millions of vaccine doses which, together, will be more than enough to give every adult in the UK two doses next year. Four million doses of the Oxford vaccine are ready to be handed over to the NHS.
Numbers of orders/pre-orders
- 100m of the Oxford-AstraZeneca vaccine
40mof the Pfizer-BioNTech
7mModerna
How does it feel to have got this far in a little under 12 months?
"It's a relief at this stage, says Gilbert, "that all of the work that people have done during the year and all of the money that's been spent on manufacturing large amounts of this vaccine, is not going to go to waste."
It was worth it! Because we worked really hard. I think we all had a little cry
On the day the results were made public, Green came home to find a colleague had left a magnum of English sparkling wine on her doorstep. But she hasn't had a chance to get together with enough people to drink it yet.
"It's a bit overwhelming to feel that the sacrifices we've made paid off and we did something big. It was worth it, because we worked really hard, I think we all had a little cry," says Green.
Pangalos hopes the vaccine means he will be able to visit his mother in Greece - they haven't seen each other since last Christmas.
The other members of the team haven't seen much of their families either, even when living in the same house. "My son said something the other day that really impacted me," says Lambe. "I was apologising for being late home from work again and he said, 'It's OK mummy, you're doing it for everybody. It's not just me you need to look after.' And that did bring a tear to my eye."
Over the course of the year it's been an emotional journey for everyone working on the vaccine,
But in the end it was the cold, hard science that really made the difference.
"I think science has been the exit strategy for this pandemic and vaccines are at the heart of that scientific endeavour," says Farrar. "It's been remarkable, there's never been a year of progress scientifically like this in my lifetime."
https://www.bbc.co.uk/news/health-55308216
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
When will the Oxford vaccine be approved?
A Covid vaccine from Oxford University and the pharmaceutical company AstraZeneca is being considered for use in the UK
It follows the roll-out of the Pfizer vaccine, which was the first to be approved.
What is the Oxford vaccine and how does it work?
It is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus - although it can't cause illness.
When the vaccine is injected into a patient, it prompts the immune system to start making antibodies and primes it to attack any coronavirus infection.
The UK has ordered 100 million doses. Unlike Pfizer's jab - which has to be kept at an extremely cold temperature (-70C) - the Oxford vaccine can be stored in a normal fridge. This makes it much easier to distribute.
How has the vaccine been produced so quickly?
Oxford University researchers had already done a lot of work before 2020 on developing a vaccine which could be adapted to tackle different diseases.
That meant a lot of the building blocks were already in place, and scientists weren't starting from scratch.
The vaccine has been through all the usual research stages, although for speed these have overlapped when they would usually happen one after another.
And the UK's medicines regulator - the MHRA - has been carrying out a "rolling review" of data all year.
When will the vaccine be ready?
It is currently in the last stage of MHRA assessment, after the government asked it to analyse the final set of data on 27 November.
Prof Sarah Gilbert, lead researcher on the vaccine, has said the chances it will be given to people before the end of the year are "pretty high".
How effective is the Oxford vaccine?
The Oxford/AstraZeneca vaccine has been shown to be safe and to provoke an immune response in people of all ages, including the over-55s.
Unlike Pfizer, which announced one figure, showing its vaccine was 90% effective, Oxford/AstraZeneca presented three different figures:
Overall, the vaccine was found to be 70% effective.
For the majority of people in the trial who got two full doses the figure was 62%.
However, for a small group who got a half dose and then a full dose, that figure was 90%. It's not clear yet why the results were better for this group.
It may be that their immune systems were primed more gradually, but the scientists can't yet explain it.
Also, all the volunteers in this smaller group were under 55.
And as with the other vaccines, scientists don't yet know if it stops people catching Covid - that's something they won't know until they can see the impact of vaccination over time.
Which dose will be recommended?
That is something the MHRA will consider.
It will keep analysing data from the ongoing Oxford/AstraZeneca research, so it could opt for two full doses initially - but rule on the half/full course once more data is available.
Is the Oxford vaccine as good as the Pfizer?
It's important to remember even the lower 62% figure is a better result than the best flu jab, which is about 50% effective.
And no-one who got the vaccine was hospitalised or got seriously ill due to Covid.
How long does it protect against Covid for?
As with all the vaccines being developed against coronavirus, we don't know yet.
It may be that people need annual vaccinations, as happens with the flu jab.
Which vaccine will I get?
Recommendations on which vaccine is given to which groups are made by the JCVI - the Joint Committee on Vaccination and Immunology - an independent group of scientists.
It was the JCVI that decided the Pfizer vaccine should first go to over-80s, health and care workers and care home residents.
https://www.bbc.co.uk/news/health-55302595
A Covid vaccine from Oxford University and the pharmaceutical company AstraZeneca is being considered for use in the UK
It follows the roll-out of the Pfizer vaccine, which was the first to be approved.
What is the Oxford vaccine and how does it work?
It is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees. It has been modified to look more like coronavirus - although it can't cause illness.
When the vaccine is injected into a patient, it prompts the immune system to start making antibodies and primes it to attack any coronavirus infection.
The UK has ordered 100 million doses. Unlike Pfizer's jab - which has to be kept at an extremely cold temperature (-70C) - the Oxford vaccine can be stored in a normal fridge. This makes it much easier to distribute.
How has the vaccine been produced so quickly?
Oxford University researchers had already done a lot of work before 2020 on developing a vaccine which could be adapted to tackle different diseases.
That meant a lot of the building blocks were already in place, and scientists weren't starting from scratch.
The vaccine has been through all the usual research stages, although for speed these have overlapped when they would usually happen one after another.
And the UK's medicines regulator - the MHRA - has been carrying out a "rolling review" of data all year.
When will the vaccine be ready?
It is currently in the last stage of MHRA assessment, after the government asked it to analyse the final set of data on 27 November.
Prof Sarah Gilbert, lead researcher on the vaccine, has said the chances it will be given to people before the end of the year are "pretty high".
How effective is the Oxford vaccine?
The Oxford/AstraZeneca vaccine has been shown to be safe and to provoke an immune response in people of all ages, including the over-55s.
Unlike Pfizer, which announced one figure, showing its vaccine was 90% effective, Oxford/AstraZeneca presented three different figures:
Overall, the vaccine was found to be 70% effective.
For the majority of people in the trial who got two full doses the figure was 62%.
However, for a small group who got a half dose and then a full dose, that figure was 90%. It's not clear yet why the results were better for this group.
It may be that their immune systems were primed more gradually, but the scientists can't yet explain it.
Also, all the volunteers in this smaller group were under 55.
And as with the other vaccines, scientists don't yet know if it stops people catching Covid - that's something they won't know until they can see the impact of vaccination over time.
Which dose will be recommended?
That is something the MHRA will consider.
It will keep analysing data from the ongoing Oxford/AstraZeneca research, so it could opt for two full doses initially - but rule on the half/full course once more data is available.
Is the Oxford vaccine as good as the Pfizer?
It's important to remember even the lower 62% figure is a better result than the best flu jab, which is about 50% effective.
And no-one who got the vaccine was hospitalised or got seriously ill due to Covid.
How long does it protect against Covid for?
As with all the vaccines being developed against coronavirus, we don't know yet.
It may be that people need annual vaccinations, as happens with the flu jab.
Which vaccine will I get?
Recommendations on which vaccine is given to which groups are made by the JCVI - the Joint Committee on Vaccination and Immunology - an independent group of scientists.
It was the JCVI that decided the Pfizer vaccine should first go to over-80s, health and care workers and care home residents.
https://www.bbc.co.uk/news/health-55302595
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Re: Coronavirus: we separate myths from facts and give advic
CHRISTMAS CANCELLED
UK PM Boris Johnson announces Tier 4 rules
Christmas bubbles have been axed for London and many surrounding areas as they were slammed into Tier 4 as a new fast-spreading variant of Covid-19 was blamed for a surge in cases
Prime Minister Boris Johnson effectively cancelled Christmas gatherings for millions of families in London and the wider South East on Saturday as he sought to contain the spread of the mutant virus.
The Christmas relaxation for the rest of the country was dramatically scaled back to three households being able to meet on Christmas Day alone rather than for five days in England.
Ministers insisted the drastic action was needed to combat the new strain of the virus VUi202012/01 which was said to have been identified first in the UK on any significant scale, with less than a handful of cases in two other countries.
Nearly 60 per cent of recent new cases in London are believed to be the new strain of the virus, with cases nearly doubling in the past week.
Mr Johnson said the new virus may be up to 70 per cent more transmissible as he announced the new Tier 4 restrictions at a somber press conference at Downing Street.
"Given the early evidence we have on this new variant of the virus, the potential risk it poses, it is with a very heavy heart I must tell you we cannot continue with Christmas as planned," he said.
Health chiefs say there is no evidence so far that it is more deadly or that vaccines may not work against it.
Mr Johnson said he was taking the actions with a “heavy heart”, but the scientific evidence which suggests the new strain was up to 70 per cent more transmissible, had left him with no choice.
“Without action the evidence suggests that infections would soar, hospitals would become overwhelmed and many thousands more would lose their lives,” he told the Downing Street news conference.
“Yes Christmas this year will be different, very different. We’re sacrificing the chance to see our loved ones this Christmas so that we have a better chance of protecting their lives, so that we can see them at future Christmases.”
Sir Keir Starmer said people were getting “confusion where they need certainty”, but reiterated that people should follow the Government guidance of their tier.
“The infection rates are going up very very fast and so something has to be done, and we support the steps the Government has put in place but millions of families are going to be heartbroken by this news – having their Christmas plans ripped up,” he said.
“I’m really frustrated because I raised this with the Prime Minister on Wednesday and he dismissed that and went on to tell people to have a merry little Christmas – only three days later to rip up their plans.
The Tier 4 rules which come into effect on Sunday will include:
Households outside Tier 4 are still being urged to limit the scale of social gatherings over the festive period, as well as the time spent together.
The new restrictions aim to slow the spread of the mutant virus to other parts of the UK.
The Government has told the World Health Organisation about its findings so far on the new variant of the virus.
Other countries may impose new bans on people travelling from the UK in the light of the development.
Health chiefs first highlighted the new variant on Monday and after further analysis the Prime Minister was given details of how fast it spreads on Friday afternoon.
Chief Medical Officer for England, Professor Chris Whitty said: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.
“Given this latest development it is now more vital than ever that the public continue to take action in their area to reduce transmission.”
https://www.standard.co.uk/news/uk/tier ... obal-en-GB
UK PM Boris Johnson announces Tier 4 rules
Christmas bubbles have been axed for London and many surrounding areas as they were slammed into Tier 4 as a new fast-spreading variant of Covid-19 was blamed for a surge in cases
Prime Minister Boris Johnson effectively cancelled Christmas gatherings for millions of families in London and the wider South East on Saturday as he sought to contain the spread of the mutant virus.
The Christmas relaxation for the rest of the country was dramatically scaled back to three households being able to meet on Christmas Day alone rather than for five days in England.
Ministers insisted the drastic action was needed to combat the new strain of the virus VUi202012/01 which was said to have been identified first in the UK on any significant scale, with less than a handful of cases in two other countries.
Nearly 60 per cent of recent new cases in London are believed to be the new strain of the virus, with cases nearly doubling in the past week.
Mr Johnson said the new virus may be up to 70 per cent more transmissible as he announced the new Tier 4 restrictions at a somber press conference at Downing Street.
"Given the early evidence we have on this new variant of the virus, the potential risk it poses, it is with a very heavy heart I must tell you we cannot continue with Christmas as planned," he said.
Health chiefs say there is no evidence so far that it is more deadly or that vaccines may not work against it.
Mr Johnson said he was taking the actions with a “heavy heart”, but the scientific evidence which suggests the new strain was up to 70 per cent more transmissible, had left him with no choice.
“Without action the evidence suggests that infections would soar, hospitals would become overwhelmed and many thousands more would lose their lives,” he told the Downing Street news conference.
“Yes Christmas this year will be different, very different. We’re sacrificing the chance to see our loved ones this Christmas so that we have a better chance of protecting their lives, so that we can see them at future Christmases.”
Sir Keir Starmer said people were getting “confusion where they need certainty”, but reiterated that people should follow the Government guidance of their tier.
“The infection rates are going up very very fast and so something has to be done, and we support the steps the Government has put in place but millions of families are going to be heartbroken by this news – having their Christmas plans ripped up,” he said.
“I’m really frustrated because I raised this with the Prime Minister on Wednesday and he dismissed that and went on to tell people to have a merry little Christmas – only three days later to rip up their plans.
The Tier 4 rules which come into effect on Sunday will include:
- * A “stay at home” message in law, apart from limited exemptions such as work, education, healthcare, childcare and exercise.
* People will be advised not to enter Tier 4 areas and Tier 4 residents must not stay overnight away from home.
* Christmas bubbles are axed so households will not be allowed to mix, apart from for support bubbles.
* Non-essential shops will have to close, effectively from close of business on Saturday, as will indoor leisure such as gyms, indoor entertainment including cinemas, bowling alleys and casinos, and the personal care sector including hairdressers and nailbars.
* Household mixing will be limited to one person being able to meet with one other person in a public space outside, with exemptions for support bubbles and for childcare bubbles and for children whose parents have separated.
* For the clinically extremely vulnerable, people who have been shielding in the past, the guidance from November will be reimposed which broadly says that these inidividuals should not to go to work, limit time outside the home and take exercise outside at less busy time.
* People currently not at home in Tier 4 will be able to travel back.
* People will not be able to travel abroad on holiday.
* Outdoor sport will be able to continue and there will be no restrictions on exercise.
* Around a third of England, in the high teens of people, will be in Tier 4.
* The areas affected are all the current Tier 3 areas in the South East including London, Kent, Buckinghamshire, Berkshire, Surrey excluding Waverley, Gosport, Havant, Portsmouth, Rother and Hastings, and East of England areas including Bedford, central Bedford, Milton Keynes, Luton, Peterborough, Hertfordshire, Essex excluding Colchester, Uttlesford and Tendring.
* Ministers reluctantly ordered the clampdown which comes despite Boris Johnson saying it would be “inhuman” to cancel Christmas.
* The rules will be reviewed in two weeks’ time, on December 30 as part of wider review of all restrictions.
* There is no impact on schools as they have already broken up.
* Police will be out enforcing the rules on New Year’s Eve.
* Talks were taking place in Scotland, Wales and Northern Ireland on whether to follow England with Tier 4.
* Tier 4 is being rushed in without being debated or voted on by MPs given the scale of the crisis.
Households outside Tier 4 are still being urged to limit the scale of social gatherings over the festive period, as well as the time spent together.
The new restrictions aim to slow the spread of the mutant virus to other parts of the UK.
The Government has told the World Health Organisation about its findings so far on the new variant of the virus.
Other countries may impose new bans on people travelling from the UK in the light of the development.
Health chiefs first highlighted the new variant on Monday and after further analysis the Prime Minister was given details of how fast it spreads on Friday afternoon.
Chief Medical Officer for England, Professor Chris Whitty said: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.
“Given this latest development it is now more vital than ever that the public continue to take action in their area to reduce transmission.”
https://www.standard.co.uk/news/uk/tier ... obal-en-GB
Good Thoughts Good Words Good Deeds
-

Anthea - Shaswar

- Donator

- Posts: 28437
- Images: 1155
- Joined: Thu Oct 18, 2012 2:13 pm
- Location: Sitting in front of computer
- Highscores: 3
- Arcade winning challenges: 6
- Has thanked: 6019 times
- Been thanked: 729 times
- Nationality: Kurd by heart
Return to Kurdistan Today News (Only News)
Who is online
Registered users: Bing [Bot]